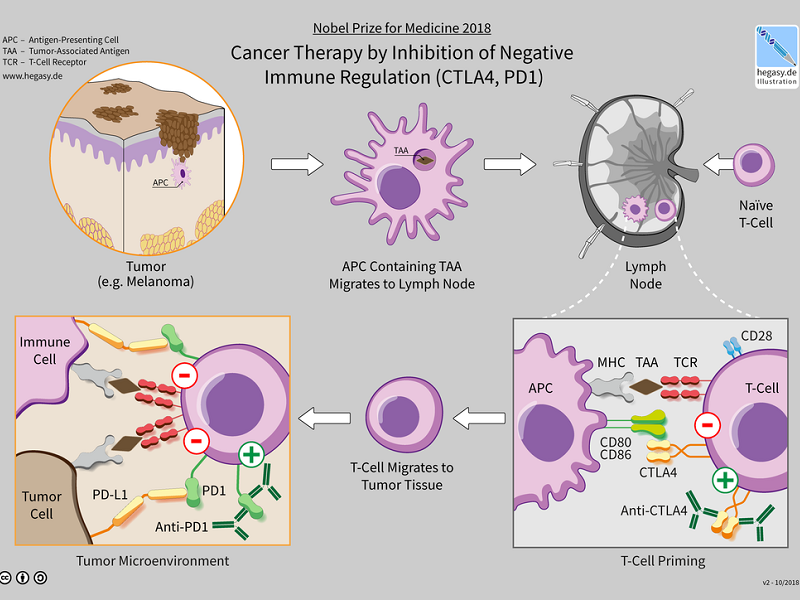
GlobalData analysed the number of clinical trials examining checkpoint inhibitor targets with start dates between January 1, 2010 and January 21, 2019. Clinical trials that investigate checkpoint inhibitor targets are pivotal for the development of cancer immunotherapies that target specific mechanisms.
The activation or inactivation of certain pathways allows for the termination of cancerous cells. Research from pre-clinical and clinical studies has shown that checkpoint inhibition can be a crucial aspect of tumour immune evasion and systemic immune suppression.
A number of clinical trials conducted on inhibitor targets seem to focus on the programmed cell death protein-1 (PD1) and programmed death-ligand 1 (PDL-1). Over the past five years, there has been a major advancement with the development of checkpoint inhibitors, with the majority of antibodies that are being studied targeting PD-1.
Overall, PD-1 had a higher percentage of clinical trials, with 62.0%, compared to the total trials for PDL-1 (23.5%) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) (18.9%), as seen in Figure 1. PD-1, PDL-1, and CTLA-4, stand out as the most valuable therapeutic targets based on the percentage of clinical trials for each target. This is due to the success of drug inhibitors targeting these checkpoints. A significant proportion of clinical trials conducted for the three targets were in Phase II (61.4%), with the majority of these trials being led by non-industry sponsors (56.6%). The US conducted 52.0% of the trials.
In 2011, Bristol-Myers Squibb’s Yervoy (ipilimumab) was approved by the FDA for targeting CTLA-4. In 2014, Merck’s Keytruda (pembrolizumab) and Bristol-Myers Squibb’s Opdivo (nivolumab) received FDA approval for targeting PD-1. In 2016, the FDA approved Genentech’s Tecentriq (atezolizumab) for targeting PDL-1.


US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData