In April 2025, the European Medicines Agency (EMA) approved Eisai and Biogen’s Leqembi for early-stage Alzheimer’s disease, marking Europe’s first major nod for a disease-modifying Alzheimer’s therapy in over a decade. This milestone underscores Europe’s cautious regulatory approach, especially when contrasted with the more expedited approval adopted by the US and Japan.
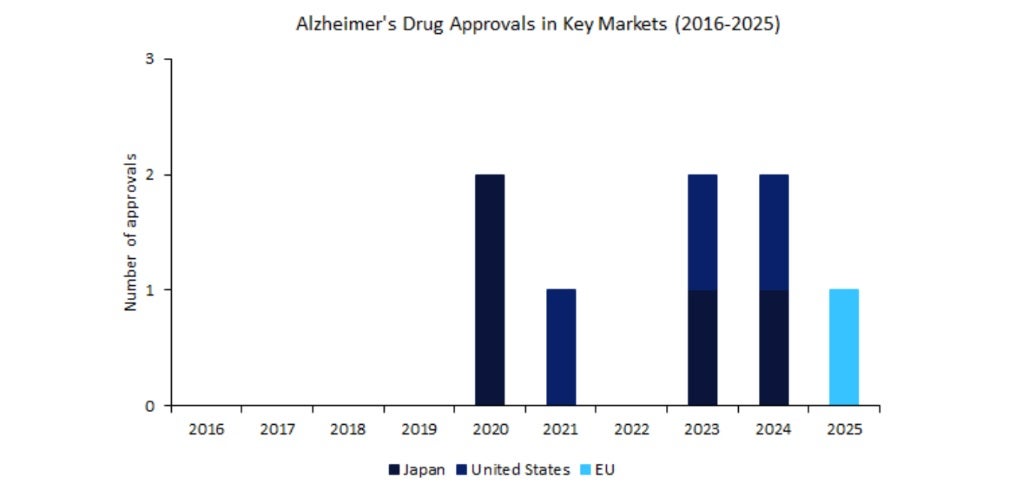
As shown in the chart above, in the past ten years, the US and Japan have collectively approved seven Alzheimer’s therapies, reflecting their more aggressive stance on accelerating the availability of disease-modifying treatments. In 2020, Japan approved two generic versions of memantine, marking a shift towards expanding treatment accessibility for patients. The US followed with approval of a major drug in 2021, Aduhelm by Biogen, and the trend continued with two additional innovator approvals in both countries – Leqembi in 2023 and Eli Lily’s Kisunla in 2024. In contrast, EMA has taken a more conservative approach, with zero approvals in the last decade until April 2025.
This contrast between regions is most clearly demonstrated by the case of Leqembi. Leqembi is a humanised monoclonal antibody that acts by selectively binding, neutralising, and eliminating soluble protofibrils, the toxic amyloid-beta aggregates. Its EU approval marked the 13th approval of the treatment worldwide, following its earlier regulatory success in other regions. After being approved in the US and Japan in 2023, it was only in April 2025, two years later, that EMA granted its approval. This was due to EMA’s citing safety and efficacy concerns, which were only overcome following additional data supporting the drug’s benefit-risk profile.
Leqembi’s approval in the EU finally opened the door to novel treatment options for patients with Alzheimer’s. However, the delay in approval in comparison to Japan and the US highlights the more conservative regulatory stance of EMA and the stricter cost-effectiveness thresholds of national healthcare systems across Europe. And EMA shows no change in this approach with Kisunla, an anti-amyloid monoclonal antibody indicated for Alzheimer’s and administered as a monthly infusion, developed by Eli Lilly, another potentially game-changing Alzheimer’s therapy. The drug has faced setbacks in Europe with EMA’s rejection in March 2025 over ongoing concerns around amyloid-related imaging abnormalities such as brain swelling and microhaemorrhages, despite having already been approved in the US and Japan.
EMA’s reluctance in approving novel drugs for Alzheimer’s underscores Europe’s prioritisation of long-term safety over rapid access. While this approach is rooted in public health caution, it can limit patient access to promising therapies at a time when disease progression remains unchecked. With Leqembi’s approval now on record, the hope is that subsequent disease-modifying drugs will not face such prolonged barriers, but the rejection of Kisunla suggests the regulatory tide has yet to fully shift.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData