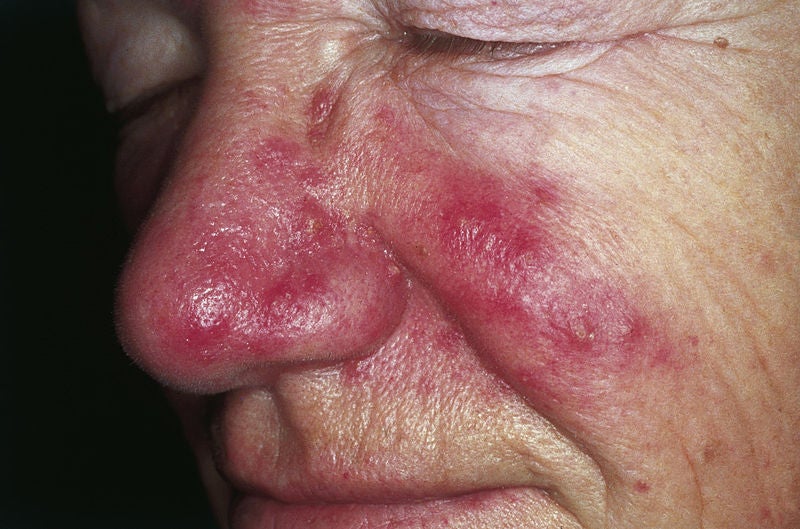
Foamix Pharmaceuticals’ FMX103 (1.5% topical minocycline foam) has experts split over the drug’s real-world ability to protect against sunburn, despite expected FDA approval for moderate-to-severe facial papulopustular rosacea.
FMX103 likely makes the skin more sensitive to sunburn, which can result in scars for this patient population already affected by pustules on their face.
No sunburns were reported in the two Phase III trials, which were combined into one 1,522-patient, randomised, double-blind, vehicle-controlled, pivotal program (NCT03142451), but patients likely had a skin care regimen such as regular use of sunscreen. Outside of a trial setting, patients will not likely put sunscreen on regularly and will be prone to sunburn over the long-term.
However, two other experts disagreed and said there is no risk of sunburn associated with FMX103, as this effect has not been reflected with generic oral minocycline. Instead, there is a risk of hyperpigmentation, they said.
Despite the potential long-term side effects, no short-term adverse events (AEs) precluded approval, experts agreed with analysts. AEs, such as upper respiratory tract infection, were seen in the Phase III data, experts said. Both the trials’ safety and positive efficacy results also support FDA approval, although the data is not clinically significant, they said.
According to a November press release, Foamix has said it hopes to file an NDA in the US in 2019.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe data’s lack of clinical significance means FMX103 will not be the first choice of treatment for papulopustular rosacea and will not replace current treatment, said one expert. Other experts said the therapy will be used as an adjunct, or alongside, therapy with an oral drug such as generic doxycycline or used after doxycycline. The compound will also be used off-label for persistent erythema rosacea (flushing), one expert said, while another said it will be used off-label for folliculitis (when hair follicles become inflamed).
Another analyst report predicted 2027 sales for $316m globally. The market cap for Foamix is $211m.
Foamix did not respond to a request for comment.
Possible hyperpigmentation and sunburn risk
FMX103 has split expert views on the likelihood of sunburn.
There may be an increased risk of sunburn outside of the trial setting, as patients may not adhere to the likely Phase III trial protocol of regular sunscreen application, said Dr Steve Feldman, professor of Dermatology, Pathology and Public Health Sciences, Wake Forest University School of Medicine, Winston-Salem, North Carolina and Dr Hadar Lev-Tov, assistant professor of dermatology, University of Miami Department of Dermatology and Cutaneous Surgery, Florida.
Oral minocycline’s prescription label states the drug causes photosensitivity, said Feldman. There is a smaller risk of sunburn when a drug is given by mouth, but the risk may increase and could cause much worse burns with a topical application, he said. Although the potential risk of sunburn does not seem to be identified in the Phase III trial, it is likely patients were advised to put on sunscreen, said Feldman and Lev-Tov.
However, there is no sunburn risk associated with FMX103, said Dr Richard Gallo, distinguished professor, Department of Dermatology, UC San Diego School of Medicine, California and Dr Helmut Schoefer, dermatovenereologist, Department of Dermatovenereology, University Hospital, Frankfurt, Germany. They pointed to years of oral minocycline being used for acne, erythema and papulopustular rosacea with no greater sunburn concerns. Although, while FMX103 has not shown any sunburn effects, there is a risk of hyperpigmentation and headaches, as seen in FMX103 data, said Schoefer and Gallo.
The two Phase III trials’ safety data identified the most common AE as upper respiratory tract infection (FX2016-11: 0.8% in the FMX103 treatment group and 2.0% in the vehicle treatment group; FX2016-12: 2.9% in the FMX103 treatment group and 3.1% in the vehicle treatment group). This data suggests that FMX103 is relatively safe, as upper respiratory tract infection is not treatment-related, said Schoefer, Feldman and Lev-Tov.
A total of nine subjects discontinued from FX2016-11 and FX2016-12 due to an AE (seven in the FMX103 treatment groups and two in the vehicle treatment groups). The discontinuations may be due to lack of compliance with the trial visits, rather than being treatment-related, said Lev-Tov.
Lack of clinically significant data hampers uptake
The Phase III trial results showed an absolute change from baseline in inflammatory lesion count at week 12 to be -17.57% for FMX103 and -15.65% for the vehicle arm (p=0.0031) for FX2016-11. The lesion reduction was -18.54% for the FMX103 and -14.88% for the vehicle arm (p<0.0001), according to the aforementioned press release.
Although this data is statistically significant and thus confirms the likelihood of FDA approval, it is not clinically significant as the absolute difference between the treatment and the placebo is minimal, said experts. The lack of clinical significance data will negatively affect market uptake, said Lev-Tov, Gallo and Schoefer. Currently, the treatment for papulopustular rosacea is first systemic, such as oral minocycline, and then a topical treatment such as generic metronidazole, experts said.
The Phase III data means that FMX103 will not be the first-line therapy for papulopustular rosacea, but rather may be used as an adjunct therapy or in combination with oral minocycline, said Gallo and Schoefer.
Due to the lack of clinically significant data, physicians will likely not prescribe it to their patients due to the assumed high costs and lack of long-term safety data, Lev-Tov said. According to public information, the cost for 50mg for a supply of 30 oral minocycline capsules is around $22, and the cost for 0.75% topical metronidazole cream is around $124.
While the target population for FMX103 is papulopustular rosacea, the unmet need is in erythema rosacea patients, as they represent a higher proportion of patients compared to papulopustular rosacea, Lev-Tov and Schoefer said. Therefore, FMX103 will likely be used more as an off-label treatment for indications such as folliculitis, said Lev-Tov.
By Arafa Salam, PhD, in London.
Arafa Salam, PhD, is a reporter for Pharmaceutical Technology parent company GlobalData’s investigative journalism team. A version of this article originally appeared on the Insights module of GlobalData’s Pharmaceutical Intelligence Center. To access more articles like this, visit GlobalData.