Amid a shortage of monkeypox vaccine doses, the WHO and FDA have declared public health emergencies, and the FDA has granted its first Emergency Use Authorization (EUA) to a monkeypox vaccine and opened the door for more.
On August 4, the US Department of Health and Human Services (HHS) declared monkeypox a public health emergency, a move required to grant EUAs. On the same day, the FDA granted a EUA to Bavarian Nordic’s (Copenhagen, Denmark) Jynneos vaccine. The drug was already approved for subcutaneous use in adults at risk of monkeypox or smallpox. But the EUA will allow more people to be treated with a smaller dose by giving it to adults intradermally, which requires only one-fifth of the volume of a subcutaneous injection. (A 2015 study showed that both routes of administration (ROA) produce similar immune responses but that intradermal ROA produced more swelling at the injection site.) The EUA also allows subcutaneous administration to individuals under 18 years old who are at high risk of monkeypox. Regardless of ROA, two doses of Jynneos should be given four weeks apart.
“In recent weeks, the monkeypox virus has continued to spread at a rate that has made it clear our current vaccine supply will not meet the current demand,” said FDA Commissioner Robert Califf. “The FDA quickly explored other scientifically appropriate options to facilitate access to the vaccine for all impacted individuals. By increasing the number of available doses, more individuals who want to be vaccinated against monkeypox will now have the opportunity to do so.”
The declaration of monkeypox as a public health emergency means the FDA may issue further EUAs for the use of unapproved vaccines or therapies or unapproved uses of approved products.
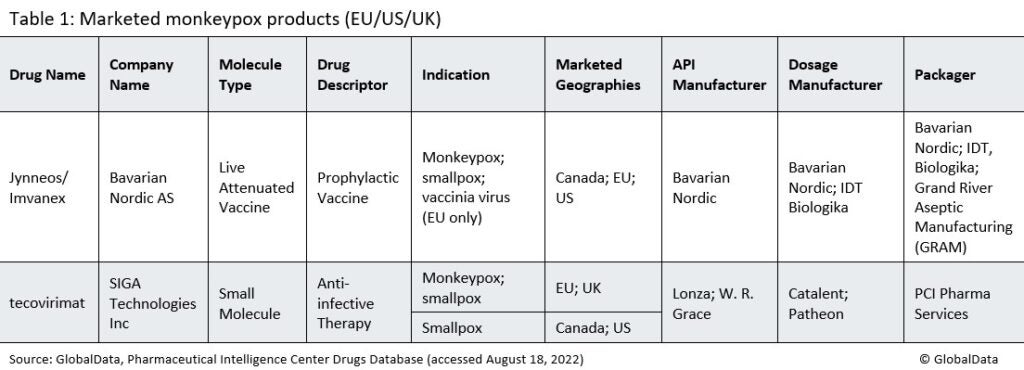
There are two monkeypox products on the market in the EU/US/UK, Bavarian Nordic’s Jynneos vaccine and SIGA Technologies’ (New York City, NY, US) anti-infective tecovirimat. (See Table 1). Japan also approved a vaccine in July 2022, marketed by KM Biologics Co (Kumamoto, Japan).
On July 26, 2022, the FDA extended the biologics license for Jynneos to allow for additional manufacturing at one of Bavarian Nordic’s plants.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe EMA expanded approved indications for Imvanex – the region’s version of Jynneos – to include monkeypox on July 22, but doses were “not immediately available,” said the EMA. Because of this shortage, EU member states have purchased 110,000 doses of Jynneos, made for the US market. “There are minor differences in terms of the manufacturing process and quality specifications between the various marketing authorizations in the different regions, which are due to differences in the datasets, but which do not affect the final quality of the vaccine,” commented the EMA.
The WHO declared monkeypox a Public Health Emergency of International Concern (PHEIC) on July 23.




