The three leading Alzheimer’s disease (AD) drugs currently in development are all monoclonal antibodies—donanemab by Eli Lilly, lecanemab by Eisai and Biogen, and gantenerumab by Roche. The rise of these monoclonal antibodies may change the dominance of small molecules within Alzheimer’s drugs, which account for 78% of all approved innovator drugs.
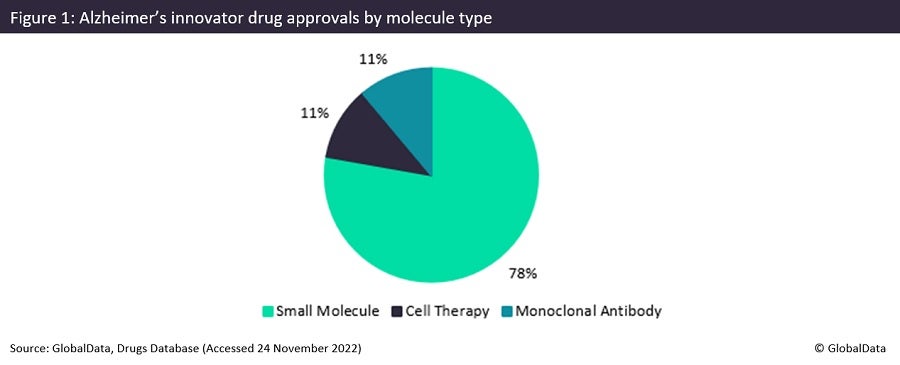
AD is the leading cause of dementia, which is an umbrella term for a series of syndromes that can lead to memory loss, cognitive decay and behavioural change. AD currently affects more than 55 million people worldwide. Over the last 30 years, there have been only nine innovative drugs approved for the treatment of AD; these include drugs such as Aricept by Eisai, a reversible inhibitor of acetylcholinesterase, which was approved in 1996, and AstroStem by Biostar Stem Cell Research Institute, a cell therapy approved in 2015. Of these nine innovative drugs, 78% have been small molecules, as shown in Figure 1, with only 22% of all AD approvals being for biologics. Monoclonal antibodies make up half of these, with an 11% share of all AD approvals.
With the significant and much-welcomed rise of the three monoclonal antibodies, donanemab, gantenerumab and lecanemab, the singular dominance of small molecules as the drug of choice for AD may be at an end. All three drugs are currently in the late stages of development, with gantenerumab in Phase III and both donanemab and lecanemab in pre-registration for the US. And with Eisai’s release of its successful Phase III trial results for lecanemab, which demonstrated a 27% slowing of neurocognitive decline in those suffering from early stages of AD, the likelihood of their approval is increasing.
The success of these drugs is not guaranteed, however, with Roche reporting on 15 November that gantenerumab had failed to meet its primary endpoint of slowing clinical decline within its Phase III trial. Furthermore, the only currently approved monoclonal antibody that has been approved in AD is the infamous Aduhelm by Biogen, which was approved by the FDA last year despite concern over its lack of any clinically meaningful benefit.
The pharmaceutical industry is shifting to focus more and more on biologic drugs and less on small molecules, evident even in the AD pipeline where monoclonal antibodies are the second largest molecule type after small molecules. The success of these monoclonal antibodies may herald a change in marketed AD drugs and create a more diverse AD drugs market that is not dominated by small molecules.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData



