Autologous cell therapy production presents an unusually difficult conflict for manufacturers and doctors, who want to get the product to the patient in time and yet need it to meet quality standards. There is very little guidance for what to do if these therapies fall slightly outside of manufacturing specifications and when to proceed with vital and often life-saving treatments. The pharma industry will need direction, and the personalised nature of the treatments will ideally require much closer communication between manufacturers and physicians.
In a CPHI Barcelona 2023 session called ‘Cell & Gene therapies: Addressing Manufacturing Challenges to Accelerate Innovation’ on 25 October 2023, Marc-Olivier Mignon, chief commercial officer at Seqens (Lyon, France), discussed the difficulties of balancing patient needs with specification requirements for cell therapies.
“There are unfortunately some situations where you are not able to manufacture the product exactly according to the specifications,” Mignon said. “But let’s say that you have specifications [of] 80% viability [from] your cells, [but] you’re [at] 78%. Then you have to make a choice because the physician calls you and says ‘Why did [the batch] fail, what’s going on?’
“We are not supposed to release the product because it’s not [meeting] specifications. And then the physician says ‘I have no time [for another manufacturing process], so give me the cells.’ And then you are pushed to deliver what we call an out-of-specification product. We used to call them ‘unidentified pharmaceutical objects’…
“And you have no idea what that means in terms of regulatory approval, legal consequences, consequences for the product, for the reputation of the product, et cetera,” Mignon concluded.
Failures and delays in cell and gene therapy production can increase the cost of these already expensive treatments. In the case of autologous cell therapies where the therapeutic cells are derived from the patient, it can result in patient mortality if the therapy is not delivered in time. Enhancing quality control processes can improve treatment outcomes, reduce costs, and increase the uptake of this relatively new class of treatments.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataStudies show that lowering the overall batch failure rate can reduce the cost of cell therapy manufacturing by millions of dollars per batch. Catching problems early significantly reduces the costs associated with hardware and instruments. Increasing the success rate of batches could lead to decreased costs for patients, or at least to more production runs per year, and therefore bring regenerative medicine to more patients. The cost of these therapies is a major impediment to their use and increasing adoption in the treatment landscape.
In the worst cases, the clinical effects of a batch failing can result in patient mortality, and the loss of product for an autologous cell therapy could result in the patient not receiving the therapy. Commercially successful autologous cell therapies that are currently being used to treat haematological cancers include Gilead Sciences Inc’s (Foster City, CA, US) Yescarta (axicabtagene ciloleucel) and Novartis’s (Basel, Switzerland) Kymriah (tisagenlecleucel). Low manufacturing capacity for Dendreon’s (Seal Beach, CA, US) autologous cellular immunotherapy agent, Provenge (sipuleucel-T), contributed to the product’s commercial failure and the company’s bankruptcy in 2015.
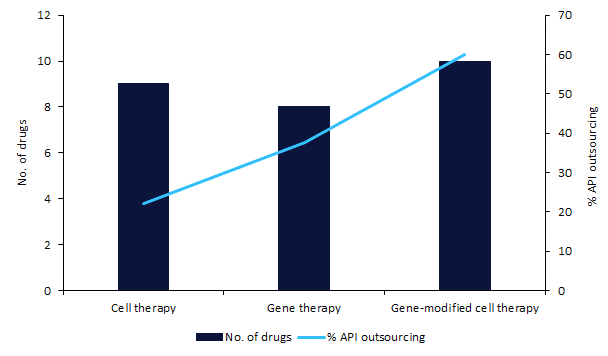
There are 27 gene-modified cell therapies, gene therapies, and cell therapies marketed in the US, UK, and/or EU. These products are outsourced to 13 different contract manufacturing organizations (CMOs) such as Catalent (Somerset, NJ, US) and Lonza (Basel, Switzerland). These CMOs will have to consider complex quality control processes for these advanced therapy medicinal products (ATMPs) at a commercial scale. API outsourcing is highest for gene-modified cell therapies, occurring for 60% of these drugs.