Intervening in apoptosis is a well understood anti-neoplastic strategy that has been shown to have efficacy in treating a number of oncology indications. However, it is increasingly clear that biological processes associated with cancer, such as uncontrolled cell division, unregulated immune responses, and excessive angiogenesis, are not unique to this therapy area. The presence of apoptosis inducers in the pipeline for oncology indications is to be expected, but scientific research has also begun to more closely examine the role of programmed cell death in diseases such as scleroderma, type 2 diabetes, and osteoarthritis.
The abundance of potential molecular targets that are involved in programmed cell death means that pharmaceutical companies can develop therapies that influence the process at any stage. One such protein is a transmembrane anti-apoptotic receptor tyrosine kinase known as the insulin-like growth factor 1 receptor (IGF1R). The receptor is activated by insulin-like growth factor 1 (IGF-1), which is a hormone that promotes the proliferation of mitosis-competent cells around the body. One example of an IGF1R antagonist is Horizon Therapeutics’ Tepezza (teprotumumab), which was the first FDA-approved drug for thyroid eye disease. Tepezza has met efficacy endpoints in multinational clinical trials for ophthalmic conditions and solid tumors.
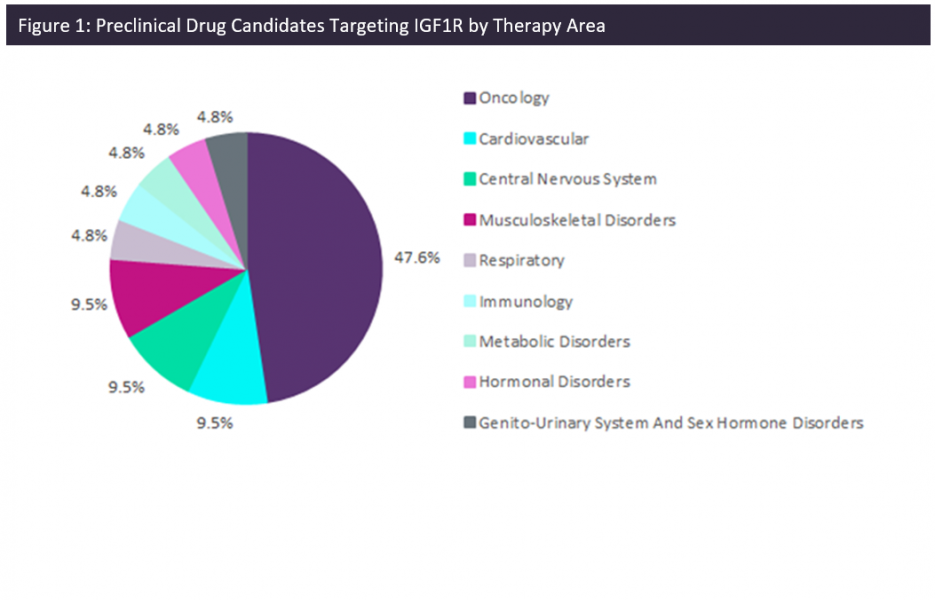
There are currently 19 drugs at the preclinical stage of development that activate or antagonize IGF1R in order to regulate apoptosis and produce therapeutic results. Whether the mechanism of action is pro-apoptotic or anti-apoptotic is indication-specific. For example, Silver Creek Pharmaceuticals’ drug candidate SGF-1 is an IGF1R agonist in development for the treatment of traumatic brain injury. The anti-apoptotic effects of this drug will promote cell survival and proliferation in an environment where damage to cerebral structures would facilitate programmed cell death. On the other hand, pro-apoptotic effects are necessary for the treatment of cancers such as glioma, breast cancer, and liver cancer which are all indications for which IGF1R-targeting products are in development. Small molecules, fusion proteins, recombinant proteins, monoclonal antibodies, and subunit vaccines are all featured among products at the preclinical stage of development.
Out of the 19 drug candidates at this stage, 68% exclusively target IGF1R. This is indicative of the importance of IGF1R in the pathophysiology of diseases in multiple therapy areas. This is expected to reduce the incidence of off-target effects, which are a common cause of adverse events. This is particularly important for inflammatory conditions such as osteoarthritis, which can be treated with steroids that are associated with negative gastrointestinal and ophthalmic outcomes.
Although most IGF1R-targeting products are in the earliest stages of clinical development, the existence of many other apoptosis-related proteins suggests that these factors may play various roles in both rare and common diseases. As such, GlobalData expects that the development of drugs that regulate apoptosis may become one of the most lucrative approaches to treat conditions outside of the oncology environment.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData