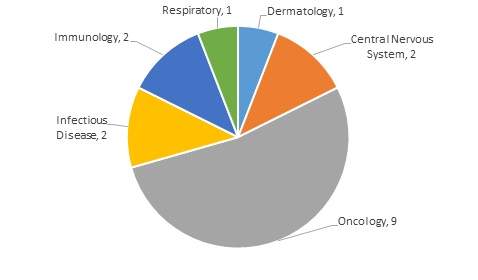
Products first approved in 2017 indicate the strength of the Oncology sector. Twenty products of the 102 FDA NDA / BLA approvals in 2017 are forecasted to sell in excess of $1bn by 2023. Of these, 9 have been approved for use in the treatment of Oncology indications.
Furthermore, of the 20 products approved for Oncology indications in the year so far, 9 are expected to breach the $1bn milestone by 2023. The oncology market is expected to see massive growth in the next decade, with the volume and favorable ratio of highly profitable approvals supporting this. With 29 additional Oncology products currently under review by the FDA, the next year is set to continue the trend.