The type 2 diabetes (T2D) market boasts a large number of available drugs and is dominated by numerous ‘me-too’ drugs, a unique feature of the T2D market due to its ability to support multiple drugs within single drug classes. The overabundance of ‘me-too’ drugs and the lack of novel agents is a major problem in the T2D space. Fortunately the early to mid-stage diabetes pipeline contains agents that belong to new drug classes.
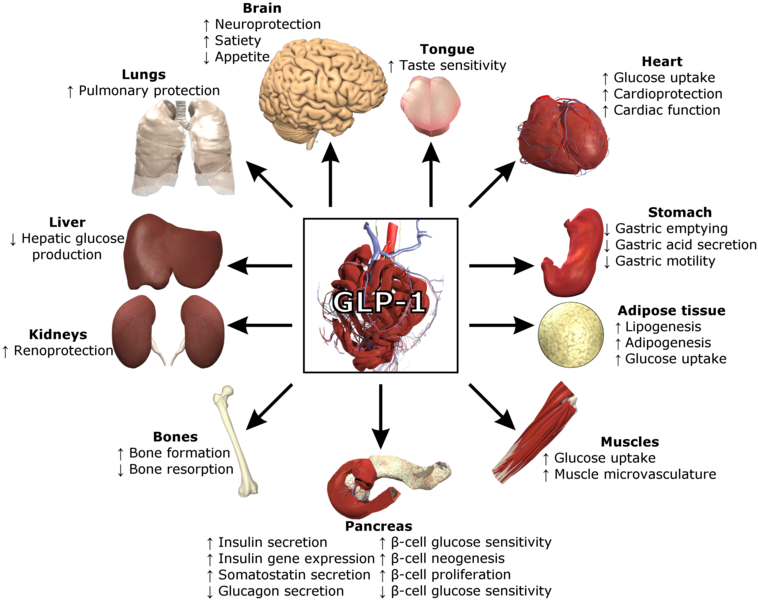
In particular, there is Eli Lilly’s LY3298176 and Sanofi’s SAR438335, in Phase II and Phase I trials, respectively. Both are unimolecular dual agents, more specifically, glucose-dependent insulinotropic polypeptide (GIP)/glucagon-like peptide-1 (GLP-1) receptor co-agonists. GLP-1 receptor agonists are already available on the market, and have become an important drug class when treating T2D.
Novel marketed GLP-1 receptor agonists, such as Eli Lilly’s Trulicity (dulaglutide), offer once weekly administration, in comparison to older GLP-1 receptor agonists which are administered once or twice per day. The drug class is effective in T2D patients because of its ability to stimulate insulin secretion, delay gastric emptying, decrease food intake, and decrease plasma glucagon.
At the 54th EASD annual meeting in Berlin, results were announced for a double-blind, placebo-controlled Phase IIb trial which enrolled T2D patients with or without metformin, treated with various doses (1mg, 5mg, 10mg, 15mg) of LY3298176. The trial had a primary outcome measure which looked at the drug’s ability to lower HbA1c compared to placebo at 26 weeks.
Secondary outcome measures focused on changes in body weight compared to placebo, the percentage of patients achieving HbA1c ≤6.5% or <7.0% at 26 weeks, the percentage of patients with ≥5% or ≥10% weight loss at 26 weeks. Secondary outcome measures also compared the change in HbA1c and body weight over time when compared to placebo and Trulicity.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataOverall, at week 26, all doses of LY3298176 treatment resulted in a statistically significant decrease in HbA1c compared to placebo, while treatment with LY3298176 doses of 5mg, 10mg, and 15mg resulted in a statistically significant decrease in HbA1c compared to Trulicity. As for weight loss, treatment with LY3298176 doses of 5mg, 10mg, and 15mg resulted in a statistically significant decrease in weight compared to placebo, while treatment with LY3298176 doses of 10mg and 15mg resulted in a statically significant decrease in weight compared to Trulicity. Side effects of LY3298176 included nausea, vomiting, and diarrhoea.
LY3298176 is made up of a GLP-1 receptor agonist combined with GIP-R activity, which resulted in an additive efficacy effect as witnessed through recent T2D clinical trials. Researchers believe this is due to GIP-R having an effect on other important tissues and systems (e.g. pancreatic islet, central nervous system, and adipose). As such, it is expected that GIP/GLP-1 receptor co-agonists represent a promising new addition for diabetic patients struggling to achieve their target blood glucose levels and body weight.
Image credit: Lthoms11.