This past Sunday, February 4, was World Cancer Day, and this year’s theme of “We can. I can.” was meant to educate the public that all individuals and groups can participate in reducing the global burden of cancer. While this is undoubtedly a tall order, many events that occurred over the past several years have involved interesting collaborations among not just traditional drug developers or professionals who treat cancer, but also those of varying disciplines.
This trend is also reflected in similar events that are set to occur later in 2018 in the healthcare industry, signifying exciting advances in cancer research and treatment. These sorts of approaches could not come at a better time, as the global incidence of many cancers is still quite high.
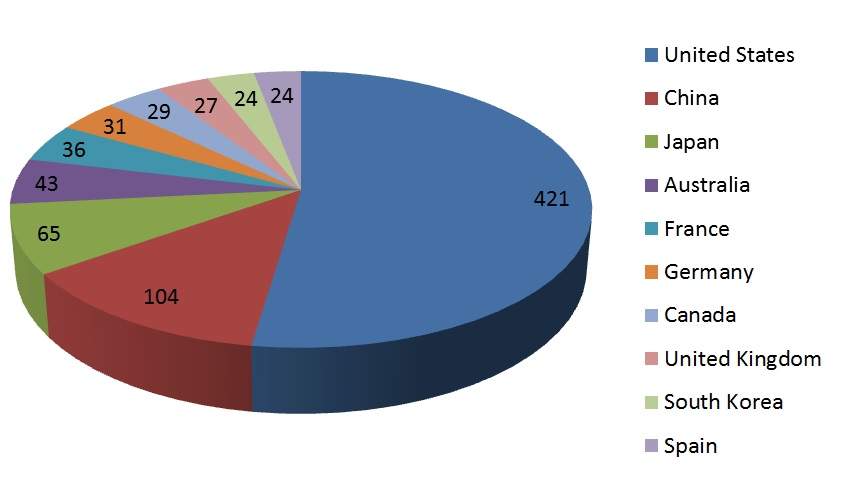
Additionally, many cancer types continue to have areas of high unmet need despite the launch of more effective and safer drug therapies for various diseases. In fact, as shown in Figure 1 below, in just the first month of 2018, a search of GlobalData’s Pharma Intelligence Center yielded over 800 clinical trials in the top ten locations worldwide, with half of these occurring in the US alone.

Figure 1: number of planned or initiated clinical trials in oncology, January 2018.
The top oncology diseases being enrolled into these trials represent a mixture of more prevalent cancers, such as breast cancer (22%) and non-small cell lung cancer (18%), with rarer forms of cancer that are characterized by greater levels of unmet needs, such as gastric cancer (10%), acute myelocytic leukemia (8%), and ovarian cancer (6%), as shown in Figure 2.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData
Figure 2: top oncology diseases in clinical trials, January 2018.
The inclusion of cancers for which there are already fairly good existing treatments and large pipelines of drugs in development, as well as those diseases for which there are not many approved therapies, indicates a good balance for cancer clinical research in terms of disease. If this trend continues, 2018 should see clinical trials results in cancers of both categories.
Cognitive computing and digital medicine tools
It is evident that in order to effectively fight the “War on Cancer” or achieve the “Cancer Moonshot”, collaborations between academia and the pharmaceutical industry are important, but so are those that are interdisciplinary in nature, such as companies involved in cognitive computing and companies developing various types of healthcare software and other digital medicine tools.
New tools that either were introduced over the past few years or will be introduced later in 2018 will continue to gain momentum and utility in diagnosing and treating cancer, including precision medicine, artificial intelligence to aid in selecting the most appropriate treatments for patients, and the use of digital technology for the reporting of information by patients during treatment.
In 2017, the results of a landmark digital medicine trial were published in the medical journal JAMA. The research reported that patients with solid tumors enrolled in a large, randomized study conducted at Memorial Sloan Kettering Cancer Center, who had access to a web-based tool to report events in real time to physicians, demonstrated a fairly large increase in overall survival compared to those who were treated with the same drug but did not report symptoms in real time.
If these significant results are seen in further clinical trial testing in 2018, GlobalData believes they could eventually trigger significant changes in the ways in which cancer patients are treated in routine practice.
Embracing new technologies
Clinical trials remain the mainstay of drug testing and enable developers to gather the evidence needed to support approval by various regulatory agencies; however, these trials contribute to a major cost of drug development. GlobalData forecasts that if drug developers start embracing the new digital advances in the coming year, this will facilitate changes in clinical trial design and outcomes, which in turn will lead to streamlined development and reduce drug development costs.
Many of these digital advances are already in the works, and include IBM’s Watson supercomputer, which is gaining traction and showing success in indicating appropriate treatments for cancer patients beyond what physicians are able to achieve on their own, as well as the Texas Advanced Computing Center, which is working to assist in drug discovery.
In addition to being used for real time, web-based reporting of symptoms by patients during clinical trials, digital tracking technology is already in use for certain approved drugs using a system developed by Witty Technology. At the end of 2017, Witty launched an additional tracking platform specifically for the newer immuno-oncology drugs that should help advance that field of treatment in 2018.
Finally, some firms are attending to cancer patients more holistically, such as CanSurround, which has developed a digital health system designed not only to reduce distress, but to increase the well-being of cancer patients. The stage is set in 2018 for digital medicine technology to have a significant impact on the cancer landscape by transforming the way cancer drugs are developed and tested for approval, as well as the ways in which cancer patients are treated during routine practice.