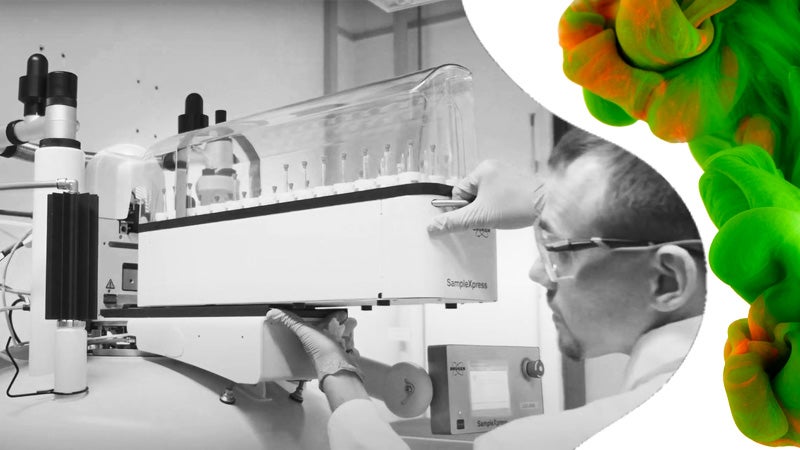
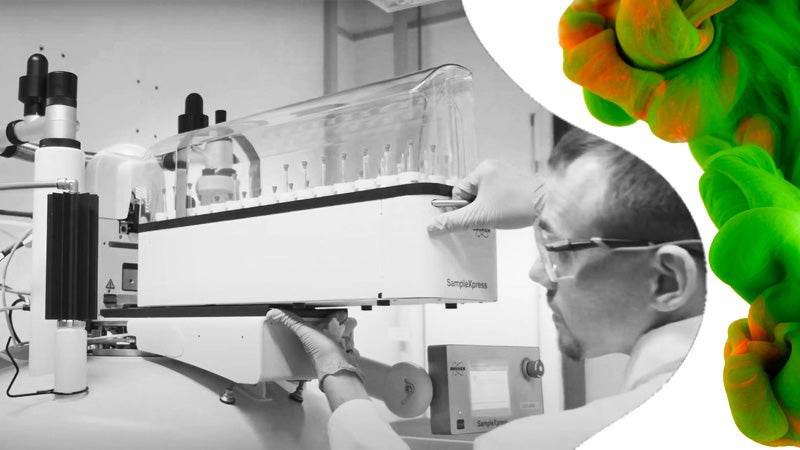
LGC is a global manufacturer and provider of reference standards. We have a long history in measurement science and producing reference materials for a wide range of applications.
Our facilities
Through a sustained programme of investment over more than a decade, LGC now has an unparalleled breadth of reference standards manufacturing capabilities.
We have manufacturing facilities around the world – in Luckenwalde, Germany; Augsburg, Germany; Manchester, New Hampshire and Charleston, South Carolina – all of which have been operating for over 20 years and are accredited to ISO 9001, ISO/IEC 17025 and ISO 17034. We have also recently opened our first reference materials manufacturing site in Nanjing, China.
Our manufacturing
LGC’s manufacturing capabilities support a large portfolio of catalogue reference standards for a wide variety of application areas, including products sold under the Dr. Ehrenstorfer™, Mikromol™, VHG™and ARMI™ brands. Alongside these catalogue ranges, we also provide a full suite of custom reference standards manufacturing services, including complete outsourcing solutions from sourcing/synthesis of materials through characterisation, certification, packaging and distribution.
Mikromol – Together, beyond the standard
Pharmaceutical reference standards and reference material services
LGC goes beyond the standard to ensure the reliability of your analysis regardless of the method used. We offer robust internal quality control, including strict release criteria and continuous quality monitoring throughout the lifecycle of standard, ISO-accredited methodology, as well as homogeneity assessments.
We provide comprehensive Certificates of Analysis (COAs) to ensure the transparency and availability of results, from 100% mass balance to volatile contents, original spectra, chromatograms, and method details. Our teams use orthogonal methods to establish identities and assays, allowing quantitative use of the standard In any method being used in your laboratory.
All our services adhere to Good Manufacturing Practice (GMP) and Good Distribution Practice (GDP) principles, as shown by our EXCiPACT® certification. We also follow stringent packaging and labelling protocols to ensure regulatory conformity.
Active Pharmaceutical Ingredient (API) primary reference standards
Mikromol’s reference standards for active pharmaceutical ingredients (APIs) are primary quantitative standards – most of them are accredited to ISO 17034 standards and designed to ensure the highest analytical accuracy and reliability. Because their intended use is for potency assessments, calibrations, drug substance and drug product release testing, they have a tight target measurement uncertainty (MU) of 0.5%.
For more information, please click here.
Excipient and Concomitant component standards
Designed to deliver the highest accuracy and reliability, Mikromol’s excipient standards are primary quantitative standards that are suitable for calibration, assay development, or as working standards. Most of the excipient products in our range are ISO 17034-accredited and all are supplied with detailed COAs featuring identity, purity, and assay data.
For more information, please click here.
Impurity reference standards
The Mikromol portfolio of more than 4,000 impurity reference standards is of the highest quality and designed to help detect degradation products in APIs and excipients, thereby ensuring they meet the limits and threshold values set by legislators and regulatory bodies.
For more information, please click here.
Custom reference standards
Mikromol understands that our clients are constantly discovering new actives and impurities of interest, so each year, our dedicated, highly-qualified customs team produces hundreds of new materials to fulfil the individual impurity profiling and API qualification needs of customers all around the world.
For more information, please click here.
Reference materials management
To support clients in bringing even safer medicines to the market, Mikromol goes far beyond supplying the right reference standards. From impurity profiling and working standards outsourcing services, to managing inventories and logistics of reference standards, we are fully equipped to provide a wide range of collaborative reference material management services.
For more information, please click here.
About Mikromol
Mikromol was founded following the fall of the Berlin Wall with the aim to provide access to tools that would enable unified quality control systems in drug release, which the East German market had lacked.
Since our foundation, the collaborative spirit that defined our start has continued through our relationships with drug manufacturers and method developers to grow our understanding of clients’ needs. We aim to offer one of the pharmaceutical industry’s broadest portfolios of reference standards and reference material services, helping companies create ever better, safer medicines.