Particle Measuring Systems Announces Corporate HQ Relocation

Particle Measuring Systems (PMS) announces the acquisition of a new building for the relocation of its global manufacturing and commercial headquarters from Airport Blvd. in Boulder, Colorado, to the Boulder Technology Center in Niwot, Colorado, USA.
The new facility is over twice the size of the current HQ at 124,000ft². It will house the manufacturing and business operations previously located in Boulder. Activities have already begun to build out the current space to meet PMS’s specific, long-term operational needs to optimize workflow and operational efficiencies across core business functions.
“This is a unique opportunity for us to design a space that accommodates the needs of all our employees while creating a positive customer and employee experience. This is a long-term strategic project for PMS and demonstrates significant capital investment by our parent company, Spectris. We are targeting operational occupancy of the building in early 2024,” said John Mitchell, President of Particle Measuring Systems. John continued, “The vision for our new PMS HQ is to create an engaging, inclusive, and welcoming workplace with employees and customers in mind.”
PMS has engaged the architecture firm DLR Group for the planning and design of the building. DLR Group has extensive experience designing mixed-use industrial manufacturing facilities.
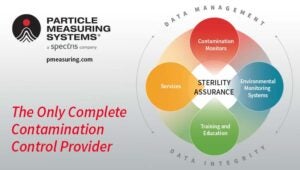
This year PMS celebrates the 50th Anniversary of its founding. Since 1972, PMS has grown into a solutions and thought leader for contamination monitoring and control for clean manufacturing facilities. They are the industry leader in monitoring sensitivity. PMS delivers not only contamination instruments, but also expert consultants, data management software, and training and education to their customers around the globe.