Nymi to Host ‘Delivering Business Value from Biometric Security’ Webinar
The demand for increased pharmaceutical manufacturing is only offset by the growing stringent requirements by regulatory bodies for good governance over the manufacturing process. Warning letters for data integrity issues have more than doubled in the last couple of years. The need for trackability and traceability is at an all-time high.
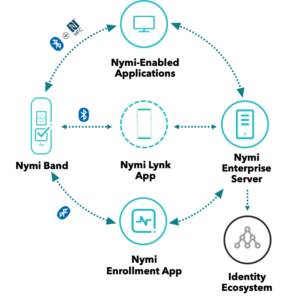
Security, data integrity and productivity are interconnected. Today’s security needs are different and more dynamic than what we’ve seen in the past. In 2018, there were more than 6,500 security breaches. Of these, 50% involved passwords.
How does a business look at security and hope to keep information secure, let alone ensure that the data is good?
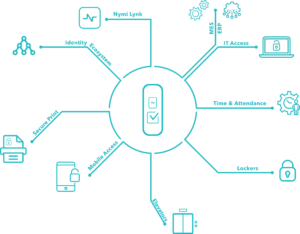
The company will look at real-world insight on how to keep create security that also allows for salient data points that can be used for traceability, trackability and compliance. It will also talk about security and productivity solutions that work in good manufacturing practice (GMP) and lab environments.
Pharma 4.0 involves improving efficiency in manufacturing. Being able to do this involves having data insight that is actionable and insightful. Joining this webinar will help you identify the factors needed in building a process that is secure, compliant and produces good data points.
This webinar will take place on 30 May at 3:00pm (GMT). Click here to register today.