Optimal Clinical Trials
Late-phase Clinical Trial Specialists
Optimal Clinical Trials provides rapid startup, high recruitment, unmatched quality and exceptional results for late-phase clinical research success.
Subscribed
You have successfully submitted your enquiry. Someone from our company will respond ASAP
About Us

Optimal Clinical Trials offers fast startup, high recruitment, and top-quality results for the success of late-phase clinical trial research.
Part of the NZCR Group, Australasia’s largest clinical trials group, Optimal Clinical Trials has earned its reputation as New Zealand’s leading late-phase clinical trials patient recruitment company by delivering results at speed and scale without ever compromising on care and quality.
This proven performance has earned the trust of many of the world’s largest and most innovative sponsors and CROs.
The Optimal Clinical Trials advantage
Optimal Clinical Trials delivers exceptional performance and reliability for sponsors and CROs worldwide.
- Speed and Scale: They frequently achieve first-patient-in and highest recruitment in Australasia and globally
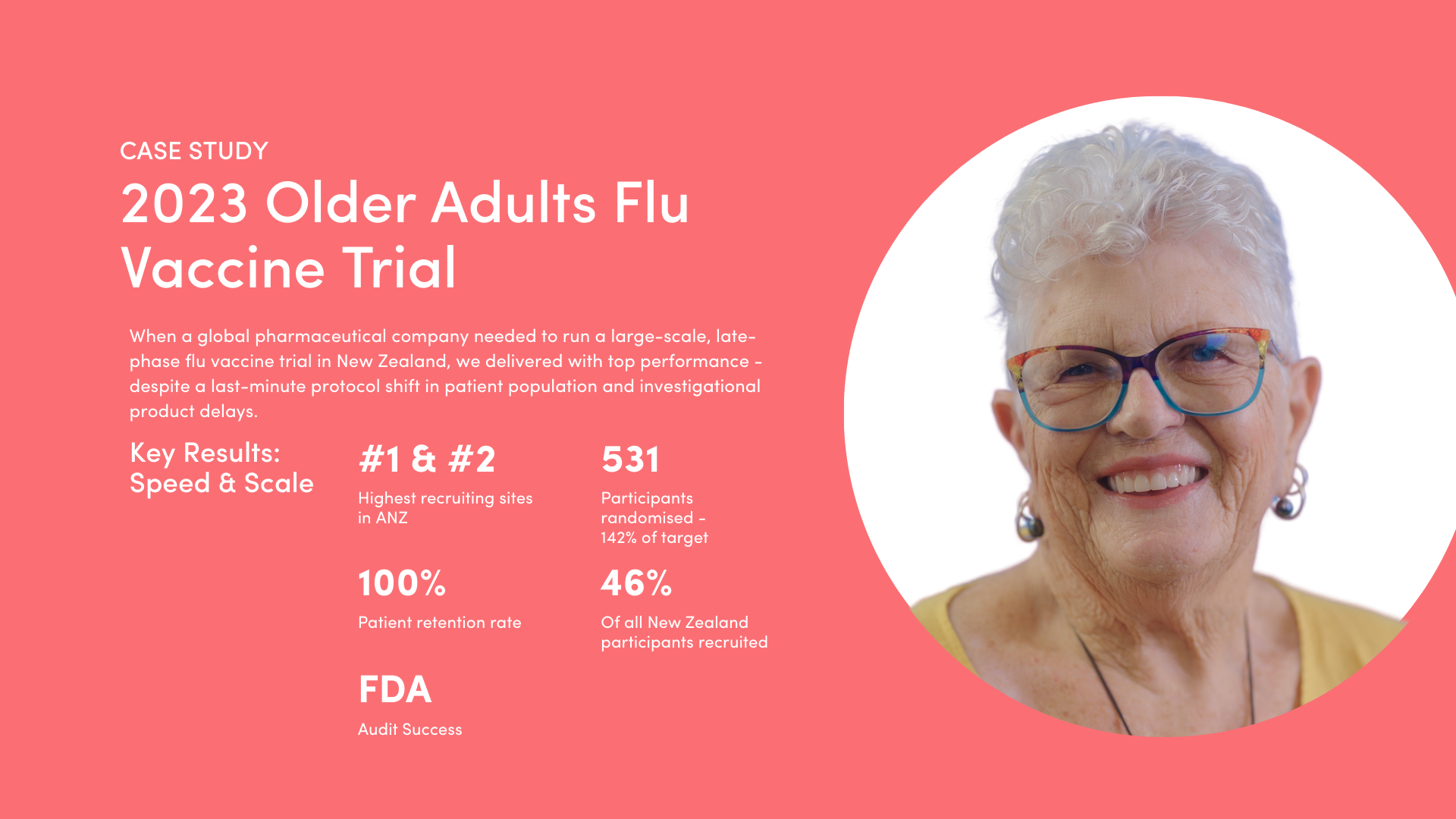
- Quality and Care: ISO 9001 certification, FDA audit success, and a Net Promoter Score of 97 reflect Optimal’s focus on quality. Sponsors gain access to KOLs, PIs and 450+ professionals across 20+ therapeutic areas, including metabolic, vaccine and older adult studies.
- People: With a people-first approach, Optimal frequently achieves 95–100% participant retention in studies. They have access to around 75% of New Zealand’s primary care database and a diverse patient pool of over 153,000 (Optimal: 60% older adults).
- Efficiency: Optimal is a government-approved RDTI provider. As a proven rescue site, they can turn around underperforming studies, enabling sponsors to achieve their recruitment goals with high-quality data, keeping trial timelines moving forward.
The late-phase clinical research site trusted by global leaders
Optimal Clinical Trials is trusted by leaders in pharmaceutical and biotech product development, along with their CRO partners.
Clients include: Pfizer, GSK, MSD, AstraZeneca, Janssen, CSL Seqirus, Takeda, Boehringer Ingelheim, Roche, Lilly, Teva, Gilead Sciences, Moderna, Novavax, BeOne Medicines, OrsoBio, Arthrosi Therapeutics, AnaptysBio, Equillium, Arrowhead Pharmaceuticals, Forte Biosciences, Axial Therapeutics, IQVIA, Syneos Health, ICON, PPD, Novotech, and HiRO.
Part of NZCR Group: New Zealand’s leading patient recruitment company for clinical trials
Optimal is a part of the NZCR Group, Australasia’s largest clinical trials group, offering sponsors complete Phase I–IV clinical trials capability.
Within the Group, NZCR and CMAX Clinical Research are leaders in early-phase studies, while Optimal and Fusion Clinical Research bring deep expertise in delivering large-scale, late-phase trials.
Together, NZCR Group’s network has successfully completed more than 1,700 clinical trials, consistently demonstrating quality, speed, and scalability for global sponsors seeking reliable trial execution.
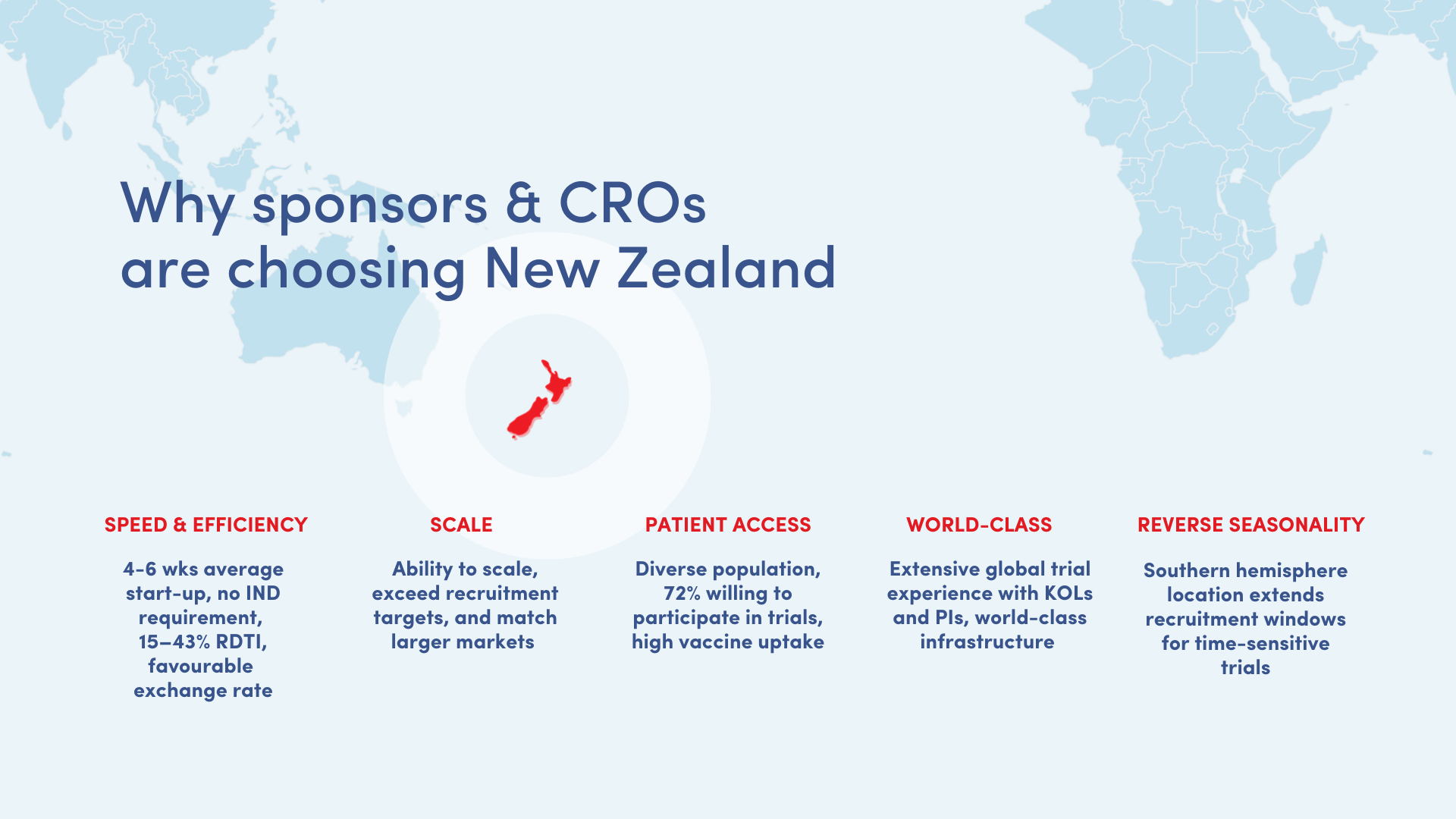
Why choose New Zealand for clinical trials?
New Zealand is a dynamic hub of innovation – examples of this include local aerospace company Rocket Lab’s space missions, studios creating Hollywood’s top visual effects, winning the America’s Cup, and developing respiratory equipment that helps millions breathe easier every year.
When it comes to clinical research, New Zealand is delivering what many sponsors assume only large, established markets can achieve. The New Zealand difference includes:
- Fast-Track Start-Up, With Fewer Barriers
New Zealand is a world leader in rapid trial start-up, with four to six weeks for regulatory and ethics approval and no IND requirement.
- Quality That Regulators Trust
New Zealand contributes frequently to global research with its skilled professionals, KOLs, and experienced PIs.
- Scale
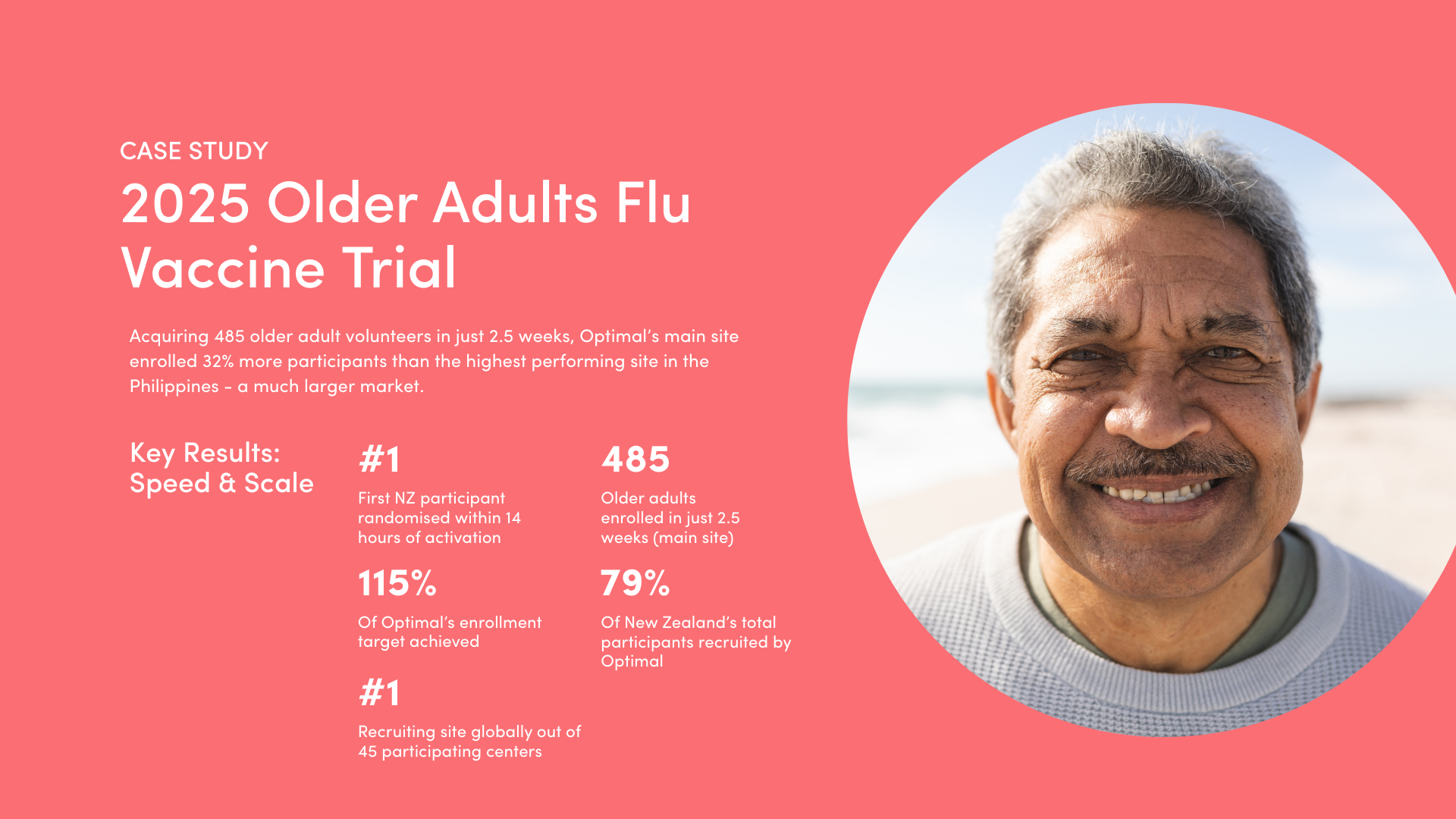
New Zealand has the ability to scale, exceed recruitment targets, and match larger markets. In a June 2025 late-phase global flu vaccine trial, New Zealand achieved 112.3% of its target, recruiting the equivalent of 95% of South Africa’s and 86% of Australia’s total enrolment.
- Diverse Population
New Zealand has a population profile similar to that of the US and Europe. With around 30% of the nation’s population born overseas, New Zealand is considered one of the most diverse countries in the world.
In addition, 72% of New Zealanders are willing to participate in clinical trials, which creates a strong foundation for rapid recruitment and robust retention.
- Vaccines Advantage
With one of the highest vaccine uptake rates globally (90% during Covid-19), New Zealand also offers a large pool for future vaccine studies. Combined with a high prevalence of major conditions and many treatment-naive patients, this creates fertile ground for both early and late-phase trials.
- Cost Efficiency
Data from PPD shows clinical trials are up to 60% less expensive to run in New Zealand than in the US, 40% cheaper than in the UK, and 10% cheaper than in Australia.
With a favourable exchange rate, running clinical trials in New Zealand creates efficiencies beyond the timeline, with compelling cost savings.
Under New Zealand’s Research & Development Tax Incentive and Tax Loss Scheme, a sponsor may be qualified to recover from 15%-43% of eligible research and development (R&D) expenditure carried out through an approved research provider (ARP) like Optimal Clinical Trials.
RDTI can be pre-approved by the New Zealand Government for one to three years, providing greater certainty.
- Trial-Ready Ecosystem
New Zealand offers a stable economy, world-class infrastructure, and reverse seasonality, which expands recruitment options.
Contact Details
Website
Email Address
Address
Auckland,
1010,
New Zealand