SCHOTT Pharma Advances Polymer Cartridge Platform for Use with SHL Medical Large-Volume Autoinjectors
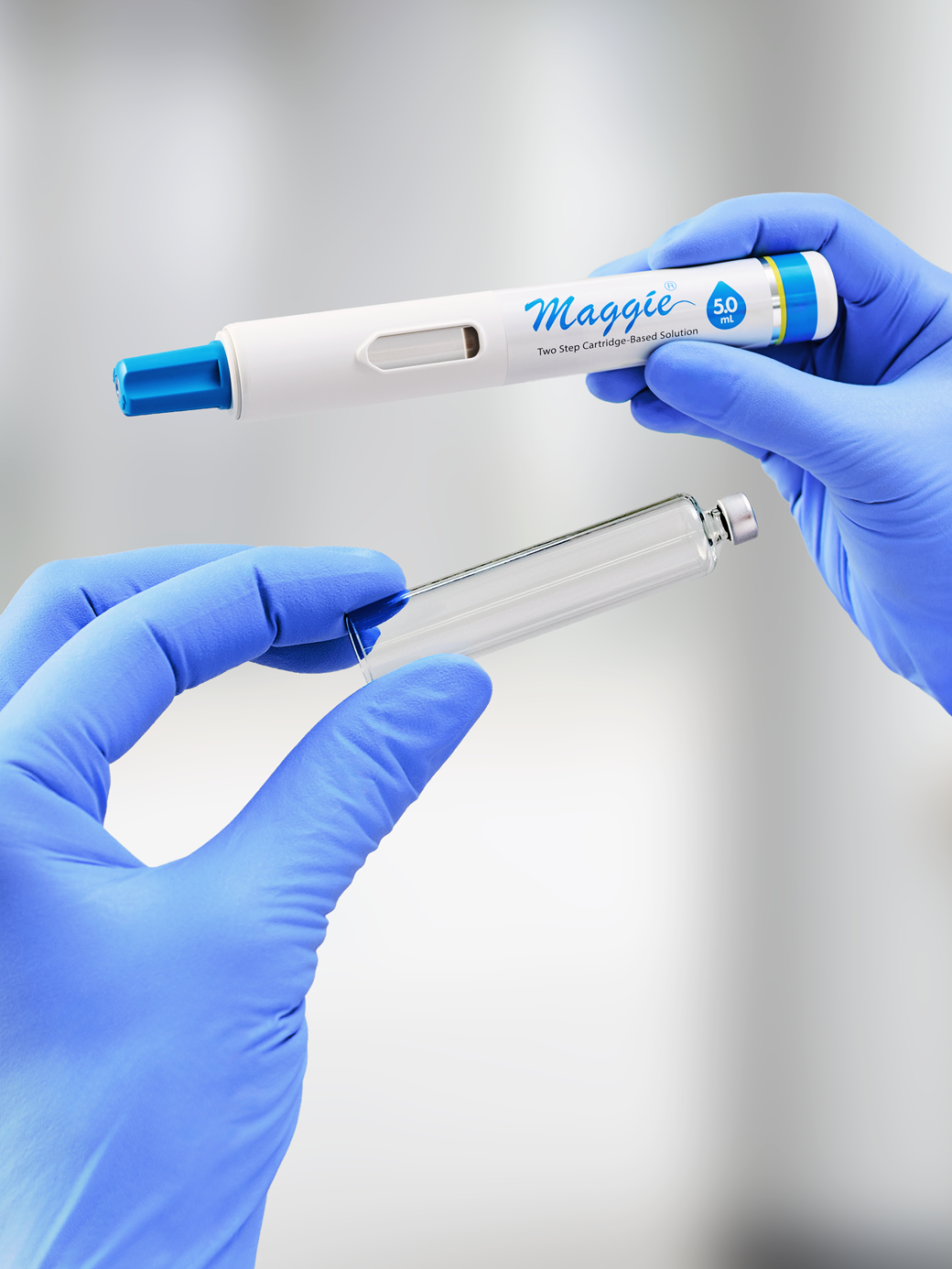
- SCHOTT TOPPAC® is the first sterile polymer cartridge platform confirmed for compatibility with SHL Medical’s Maggie® large-volume autoinjector family
- First ISO-dimension, ready-to-use (RTU) polymer cartridge offered within the SCHOTT iQ® standard—developed for large-volume delivery of highly sensitive biologics
- Additional device evaluations are in progress to support more integrated, end-to-end drug delivery combinations
SCHOTT Pharma, a provider of drug containment and delivery solutions, is strengthening its collaboration with SHL Medical by completing compatibility pre-validation between the SCHOTT TOPPAC® polymer cartridge platform and SHL Medical’s Maggie® large-volume autoinjectors. The work supports pharmaceutical customers seeking pre-assessed combinations of primary packaging and devices for home administration of larger injection volumes.
The SCHOTT TOPPAC® polymer cartridge is the first polymer cartridge in SCHOTT Pharma’s portfolio designed to align with ISO cartridge dimensions while also being supplied as a ready-to-use (RTU) product under the company’s SCHOTT iQ® standard. Polymer containers can be advantageous for drug products with specific needs—such as reduced silicone exposure or deep-cold storage conditions—and are intended for sensitive formulations, including biologics, cell and gene therapies, biosimilars, and certain emergency medicines.
“Together with SHL Medical, we’re moving closer to a more complete, de-risked offering for pharmaceutical companies—combining primary packaging and delivery device considerations early on,” says Andreas Reisse, CEO of SCHOTT Pharma.
The jointly developed and pre-validated combination will be presented at Pharmapack Paris (January 21–22), Hall 4, Booth G38.
Compatibility pre-validation results
SCHOTT Pharma and SHL Medical evaluated both dimensional integration and functional performance of the cartridge–device combination. The studies confirmed compatibility for:
- 3mL SCHOTT TOPPAC® polymer cartridge with Maggie® 3.0 mL autoinjector
- 5mL SCHOTT TOPPAC® polymer cartridge with Maggie® 5.0 mL autoinjector
This provides pharmaceutical companies with a pre-assessed system intended to reduce development risk and support safe, convenient self-injection of large-volume therapies outside clinical settings.
As part of the SCHOTT iQ® platform, the polymer cartridges are delivered in a widely adopted nest-and-tub configuration, helping simplify fill-and-finish operations and supporting faster industrialisation.
For customers evaluating different container materials, SCHOTT Pharma also notes that its 3 mL and 5 mL cartriQ® glass cartridges are compatible with Maggie® 3.0 mL and 5.0 mL autoinjectors as well—enabling selection based on product requirements and patient needs.
Next steps: broader device options
Building on the Maggie® milestone, the partners plan further work to assess SCHOTT TOPPAC® polymer cartridge compatibility with SHL Medical pen systems. SCHOTT Pharma is also evaluating additional opportunities to expand compatibility across other device platforms.
“Our collaboration with SCHOTT Pharma helps accelerate innovation and execution,” says Markus Puusepp, Chief Growth Officer at SHL Medical. “We look forward to continuing this work and bringing the benefits of the SCHOTT TOPPAC® cartridge together with our injection systems to customers and patients.”