NextPharma San Diego Facility
NextPharma's San Diego facility in California, USA, is a cGMP contract manufacturing facility serving companies in the b

NextPharma is a leading pharmaceutical contract development and manufacturing organisation providing a flexible, responsive service to markets worldwide.
You have successfully submitted your enquiry. Someone from our company will respond ASAP
NextPharma is a leading pharmaceutical contract development and manufacturing organisation providing a flexible, responsive service to markets worldwide.
NextPharma develops and produces a broad range of pharmaceutical dosage forms:
We are also specialists in:
Our FDA-approved facility in Waltrop (Germany) is a long-time centre of excellence in the development, manufacturing and packaging of hormonal drugs.
Our packaging capabilities include specialised blistering and filling into bottles of tablets, hardgel capsules and softgel capsules containing sexual hormones.
Ointments, creams and gels manufactured can be filled in either aluminium tubes, aluminium canisters or stick packs.
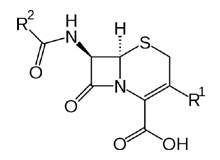
With dedicated facilities for both cephalosporins and penicillins, NextPharma has a strong history in development and supply to global customers with solid (tablets, capsules, sachets and powder dry syrups) or liquid form capabilities and expertise.
We have specific climatic requirements to ensure production of amoxicillin-clavulanic acid drugs and our packaging lines can ensure alu-alu blistering
NextPharma offers extrusion, as well as layering on non-pareils for pellet manufacturing technologies.
Extrusion pelleting allows for compact high-density pellets, resulting in optimal API content and smaller capsule sizes with the same API dose, versus layering on non-pareils.
Within the different NextPharma facilities, capabilities and expertise are available to handle narcotic drug products for pharmaceutical development, clinical trials services, commercial manufacturing and packaging. We can store and handle narcotics of the highest security category.
Why partner with NextPharma?
Contact us to discuss how we can support your goals.
NextPharma – Smart Every Time

NextPharma's San Diego facility in California, USA, is a cGMP contract manufacturing facility serving companies in the b

UK-based contract manufacturer NextPharma's plant in Braine-l'Alleud, Belgium specialises in the production and packagin
NextPharma's Clinical Trial Services (CTS) facility in Gottingen, Germany, launched in December 2008. The facility is an

The Bioavailability / Bioequivalence, Dissolution and Biowaivers Conference is one of the leading conferences for clinical, R&D and regulatory teams working with bioequivalence studies, biowaivers and dissolution testing.

NextPharma Technologies Holding Limited, a leading provider of contract development and commercial manufacturing services to the global pharmaceutical industry, today announced the appointment of two new site managers and a group VP Quality and Regulatory.