Ypsomed Receives FDA Clearance for SmartPilot
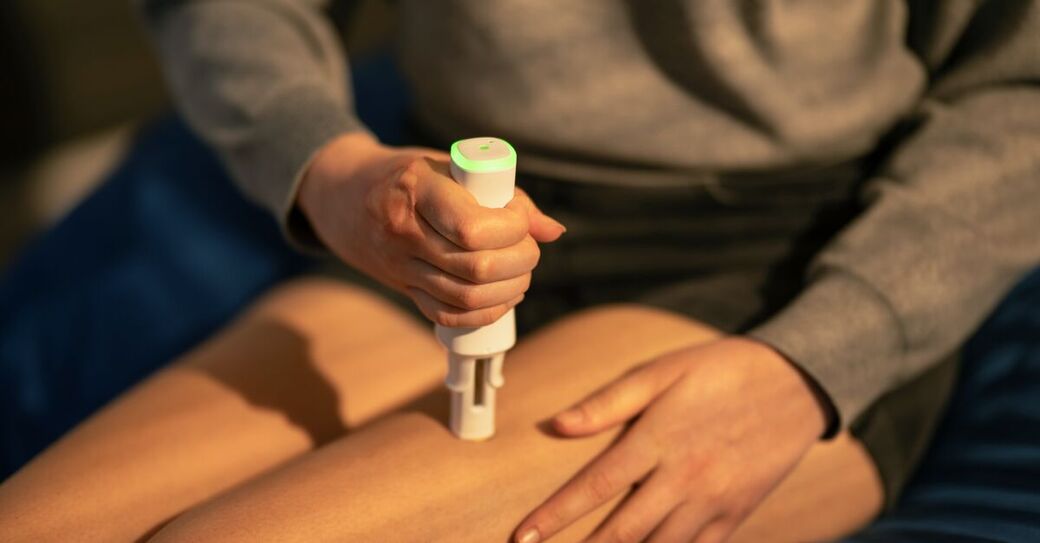
The US Food and Drug Administration (FDA) has granted 510(k) clearance for SmartPilot, a digital connectivity add-on developed by Ypsomed. The device transforms the YpsoMate autoinjector into a connected system that automatically captures and transmits injection data. The clearance marks an important step toward digital health solutions that offer deeper insights into therapy use, enable objective monitoring, and support improved adherence and treatment outcomes.
“The FDA clearance of SmartPilot authorises the device to be legally marketed and demonstrates its readiness to support our partners in both clinical trials and commercial applications. This is an important step forward in advancing digital technologies for self-injection and self-care. It validates our commitment to intelligent, patient-centred design and strengthens our partnerships with pharmaceutical companies and digital health partners,” says Ulrike Bauer, chief business officer.
Enabling digital therapy management
SmartPilot captures key injection data – such as time and date, injection outcome, and potential user errors – and transmits it securely via Bluetooth to patients’ smartphones. By enabling automatic and real-time data collection, the device supports both clinical development and day-to-day therapy management. SmartPilot is the first and only FDA-cleared connectivity device specifically designed for use with an autoinjector platform. Until now, all FDA-cleared devices for capturing injection data have been limited to pen injectors, primarily used in diabetes treatment. This makes SmartPilot a pioneering technology for broader therapy areas.
SmartPilot is compatible with subcutaneously administered medications, including both small molecules and biologics, the latter increasingly used to treat chronic and complex conditions. These medications have a range of applications that span multiple therapeutic areas, such as autoimmune diseases, cardiovascular conditions, obesity, hormone disorders, neurological and oncological indications, and rare diseases.
Benefits for patients, healthcare providers, and industry
Patients are guided through each injection step via visual and acoustic signals. SmartPilot detects and identifies user errors and logs all relevant injection data, helping improve the safety and reliability of self-injection. Step-by-step use instructions, real-time feedback, and automatic data capture reduce stress and build confidence. For healthcare professionals, access to real-time injection data enables timely intervention and better-informed treatment decisions. For pharmaceutical companies, SmartPilot offers a scalable digital platform that supports adaptive clinical trial design, market introduction, and individualised care strategies.
Implementation and outlook
Ypsomed is working closely with digital therapy management providers, as well as pharmaceutical and biotech partners, to integrate SmartPilot into clinical studies and commercial programmes. The goal is to make self-care simpler, safer, and data-driven – contributing to more integrated, effective, and patient-centred healthcare.
Disclaimer
SmartPilot is intended for the capture and recording of injection data from compatible autoinjectors to provide comprehensive user feedback using visual indicators and audible signals to guide users throughout the injection process. It is not specifically indicated for the treatment, prevention, cure, or diagnosis of a disease or condition. Although SmartPilot may enhance the user experience, the therapeutic effect is provided solely through the use of the delivered drug under the supervision of a qualified healthcare provider. If you have questions about the SmartPilot, your treatment, or your medical condition, please consult a qualified healthcare professional.