Transform Your Spectroscopic Analysis with Lyza 7000 FTIR Spectrometer from Anton Paar
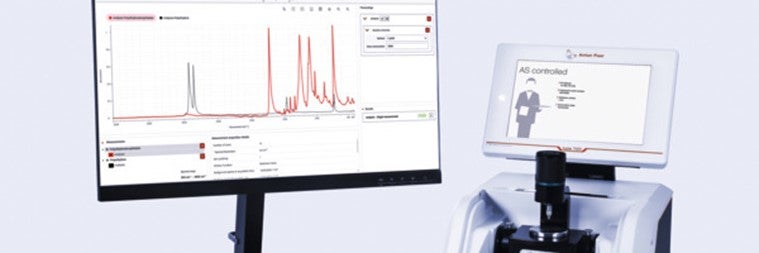
Efficiency Meets Compliance in Spectroscopy
Anton Paar, a leader in precise laboratory instruments, announces the launch of the Anton Paar Spectroscopy Suite for Lyza 7000 FTIR spectrometers. This software enhances spectroscopic analysis, combining efficiency with compliance to streamline quality control in regulated environments.
The Anton Paar Spectroscopy Suite, designed for flexibility and high data integrity, simplifies complex processes through predefined workflows, making it user-friendly and effective for rapid, reliable results. It supports operation via the PC and integrates seamlessly with both Lyza 7000 FTIR and Cora 5001 Raman, thereby setting a new standard in spectroscopy technology.
Key Features:
- Efficiency and compliance: The suite’s interface allows for easy navigation, tailored to support stringent regulatory standards, including 21 CFR Part 11 and EU GMP Annex 11.
- Data integrity: Ensures lifetime data accuracy, crucial for meeting audit requirements.
- Quality control: Enables identification of raw materials to prevent batch contamination and verifies the chemical composition of final products.
- Pharma qualification package: Accelerates instrument qualification with a comprehensive package including DQ, IQ, OQ, PQ, risk analysis, and a checklist for compliance.
- Data management integration: The suite is compatible with AP Connect, Anton Paar’s data management software, which enhances laboratory productivity by centralizing data.
Anton Paar Spectroscopy Suite not only boosts the operational capabilities of Lyza 7000 and Cora 5001, but also ensures laboratories can meet the highest standards of quality and compliance with ease.
Application report
Identify testing of raw materials using Lyza 7000 and Spectroscopy Suite: Active pharmaceutical ingredients.
The Lyza 7000 FTIR Spectrometer, coupled with the pharma-compliant Anton Paar Spectroscopy Suite software, offers a precise and efficient solution for the analysis of raw materials, fully compliant with international pharmacopoeias. FTIR identity testing is described in this report by the example of ascorbic acid (vitamin C). Download here.
Find out more by visiting our website or contacting us via email info.uk@anton-paar.com.