RSD is a European company that manufactures and develops industrial sterilisation solutions for medical device and pharmaceutical companies.
The company specialises in manufacturing ethylene oxide (EO) sterilisers and offers turnkey EO equipment sterilisation and ancillary equipment.
RSD’s turnkey solutions are efficient and innovative sterilisation facilities that use advanced technology and comply with the safety levels and regulatory requirements of the medical industries.
Ethylene oxide sterilisation equipment and turnkey solutions
RSD utilises its experience of the EO sterilisation process to provide high-quality sterilisation services and customisable equipment. It adapts these solutions to meet client specifications and provides project support throughout projects, from initial phases to completion.
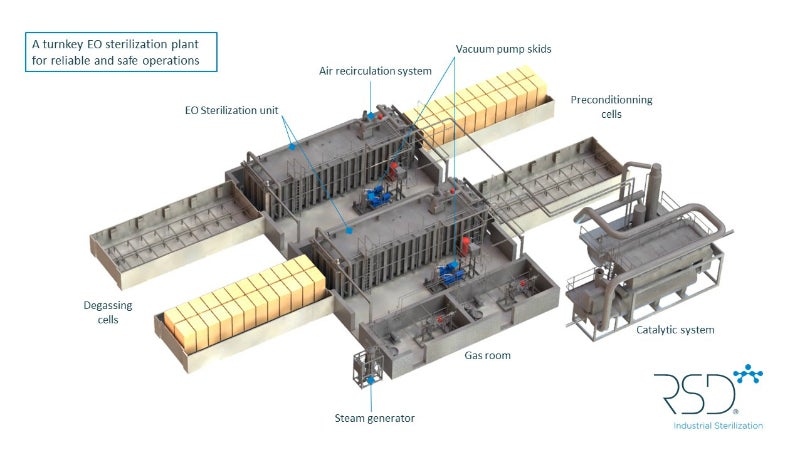
The company’s portfolio includes EO sterilisers, steam generators, EO detection systems, and automatic loading and unloading systems. These can be selected individually or combined as part of a turnkey EO sterilisation project. A wide range of options and configurations are available.
RSD manages all project phases, including design, development, manufacturing, control, commissioning and validation.
High-efficiency customisable EO steriliser and autoclave design and manufacturing
RSD’s modular industrial sterilisers have a flexible design to help divide high-risk processes and reduce the probability of developing an explosive atmosphere during EO sterilisation.
They also maintain a uniform EO concentration and temperature, have high levels of safety (ATEX certified for zone1 and zone2) and are optimised for energy consumption and cycle time.
The systems include gas rooms, vapouriser skids, vacuum pump skids and control room as standard. They also have further options for parametrical release, automatic loading and unloading systems, preconditioning and degassing cells, steam generators, catalytic systems or scrubbers and EO detectors.
They are also customisable and use both 100% EO (+N2) and a 90/100 mixture to limit the chamber’s internal pressure (<+0,5b).
RSD’s EO sterilisation chambers can work all-in-one or with additional preconditioning and degassing cells.
EO sterilisation equipment safety
RSD’s units are rigorously tested to ensure a high level of safety for both the equipment and its operators.
The sterilisers also use a programmable logic controller (PLC) comprising special safety modules that are programmed together with a supervisory control and data acquisition (SCADA) unit, according to Hazard and Operational Stability study (HAZOP).
In addition, safety integrity level 2 (SIL2) is applied to instrumentation, steriliser components and the PLC.
Qualification of sterilisation equipment
RSD conducts a wide range of validation and qualification tests to optimise sterilisation equipment operations to avoid risk. Stringent protocols are used, including:
- Design qualification (DQ), including functional design qualification (FDS), safety data sheets (SDS), safety monitoring device and system (SMDS)
- Factory acceptance tests (FAT) and site acceptance tests (SAT)
- Installation qualification (IQ) and operational qualification (OQ)
- Support for performance qualification (PQ)
The sterilisers and ancillary equipment are CE certified and comply with European standards and directives. Training for operators and maintenance staff is included in RSD’s turnkey projects.
Pharmaceutical engineering for industrial equipment
As experts in industrial sterilisation, RSD provides engineering services for sterilisation equipment such as pure steam, superheated water, air+steam sterilisation systems and other pharmaceutical equipment.
The company handles layout, 3D design and constructive drawings; quality control and project management aspects. Its specialists are Ism-ATEX certificated level 1 and 2 for design and maintenance of both electrical and mechanical systems.
Consultancy services for EO sterilisation process
RSD offers consulting services for EO cycle optimisation and validation, maintenance of autoclave sterilisation and plants, installation and verification. They also provide consultancy solutions for qualification protocols and tests, plant scale-up, utility calculations, safety audits according to the ATEX 2014/34/UE directive, installation upgrades and parametric release implantation.
Please use the contact form to send RSD your business enquiry.