Crossing the approval hurdle for a new drug is a big milestone for pharmaceutical companies. However, efficiently manufacturing the drug represents another barrier to cross before realizing the full revenue potential then successfully.
Each month, Pharmaceutical Technology takes a look at recent decisions taken by regulatory and reimbursement agencies and identifies the key manufacturing players that can be impacted by them. Contract manufacturing organizations (CMOs) are critical to ensuring the adequate and timely production of drugs and are involved in each step of the way.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
For this edition, Pharmaceutical Technology considers the regulatory decisions from late September through early November. This data breakdown is based on the GlobalData Pharma Intelligence Center’s Deals database and PharmSource reports. GlobalData is the parent company of Pharmaceutical Technology. These contracts involve the manufacturing of biological and small molecule active pharmaceutical ingredients (APIs), parenteral manufacturing and packaging, and other manufacturing-related tasks.
Regulatory decisions come in
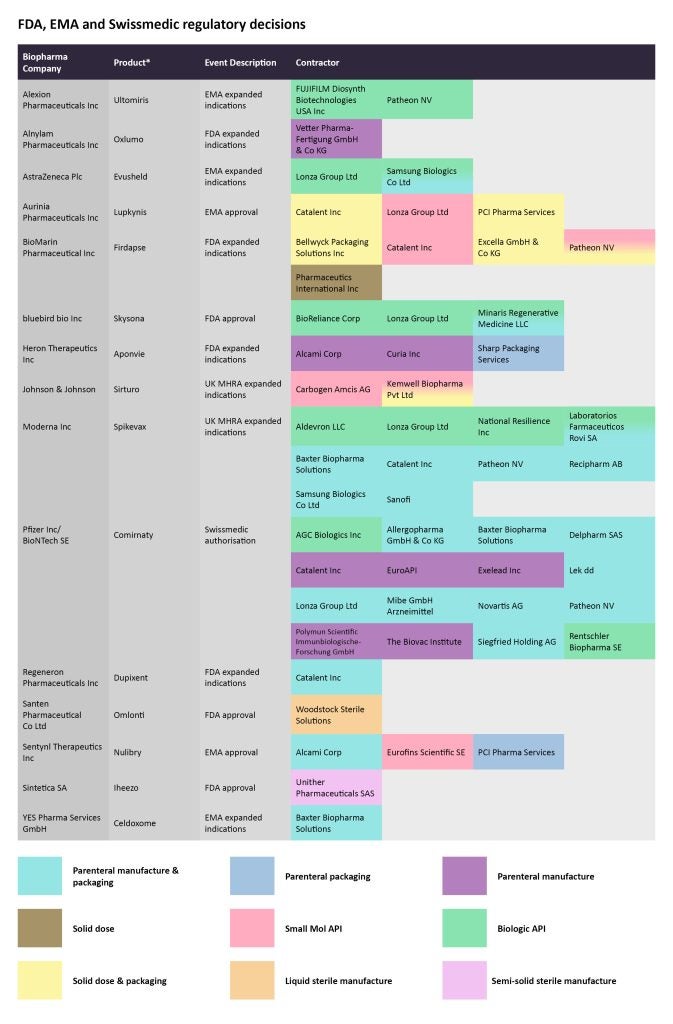
In late September, the European Commission approved the rare disease therapy Ultomiris (ravulizumab), developed by Alexion, a division of AstraZeneca to treat generalized myasthenia gravis. The EMA had provided a positive opinion for this marketing authorization back in July. The monoclonal antibody (mAb) is already approved for treating paroxysmal nocturnal haemoglobinuria in children and adolescents. Fujifilm Diosynth Biotechnologies and Patheon, by ThermoFisher Scientific, are tasked with producing the biological API for this therapy.
On September 16, another rare disease therapy, bluebird bio’s Skysona (elivaldogene autotemcel), received FDA approval to treat early, active cerebral adrenoleukodystrophy (CALD) in boys between the ages of four and 17. The biological API manufacturing has been outsourced to BioReliance Corp, Lonza, and Minaris Regenerative Medicine, while Minaris is also assigned the parenteral manufacturing and packaging for Skysona.
Source: GlobalData Pharmaceutical Intelligence Center
On the same day, the EMA approved AstraZeneca’s COVID-19 long-acting antibody combination Evusheld (tixagevimab and cilgavimab), for treating adults and adolescents at an increased risk of progressing to severe COVID-19 but not requiring supplemental oxygen. However, a few weeks ago, the UK’s National Institute for Health and Care Excellence (NICE) did not recommend five major COVID-19 therapies, including Evusheld, in a draft guidance. While the Lonza Group and Samsung Biologics are manufacturing the biological API, the latter is also in charge of the parenteral manufacture and packaging.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataFollowing similar decisions by the American and European regulators, Swissmedic approved Pfizer/BioNTech’s bivalent Comirnaty booster. Several companies have been tasked to do the parenteral manufacturing and packaging for the vaccine including Allegropharma GmbH; Baxter Biopharma Solutions; Delpharm; Lek dd, a Sandoz company; Lonza; Mibe GmbH Arzneimittel; Novartis; Patheon; and Siegfried Holding.
Also in September, the US FDA expanded Sanofi/Regeneron Pharmaceuticals’ Dupixent (dupilumab) label to include prurigo nodularis. Catalent has been engaged in the parenteral manufacturing and packaging of the anti-IL-4/IL-13 antibody.
NICE decisions
The UK’s NICE provides guidance on the cost effectiveness of new treatments; the guidcance is taken into account for reimbursement and coverage decisions by the UK’s National Health Service. In the case of Bristol Myers Squibb’s Zeposia (ozanimod), the authority supported its use to treat moderate to severe ulcerative colitis. For Zeposia, Carbogen and Patheon are producing the small molecule API, while the latter is also in charge of solid dose manufacturing and packaging.

Source: GlobalData Pharmaceutical Intelligence Center
The agency did not support the use of Takeda Pharmaceuticals’ Entyvio (vedolizumab) for chronic refractory pouchitis after surgery for ulcerative colitis in adults, citing the fact that the company did not submit evidence for the same. AbbVie and Lonza are producing the biological API, while Delpharm is tasked with the parental manufacturing.
To read the previous editions in this series, click here, here and here.




