
Four weeks into her pregnancy, Jessica Intermill sensed something was wrong when the pain in her fingers wouldn’t subside. What followed was a months-long cycle of specialist visits before a rheumatologist finally accepted that her constant joint pain was not simply a pregnancy-related issue. Intermill received a diagnosis for the autoimmune disease rheumatoid arthritis—setting off a long and complicated journey to receive treatment.
Throughout her pregnancy and postpartum, Intermill navigated insurance technicalities, including prior authorisation and step edits, and was placed on older, less effective drugs such as methotrexate, before she was able to access an effective biologic.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Since then, Intermill has been on a biologic that has been effective in treating her condition for several years. Her experience navigating drug prices and the fear of losing access due to financial or insurance reasons prompted her to speak at a recent meeting of a Prescription Drug Affordability Board (PDAB) convened by the state of Minnesota, where she is a resident.
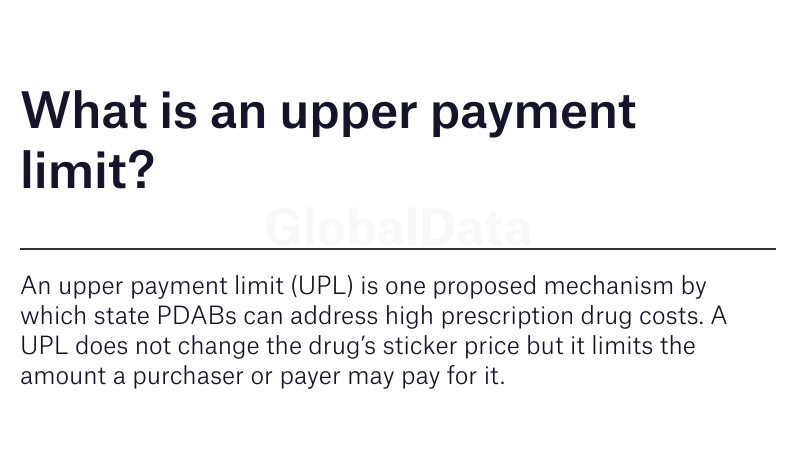
Minnesota is among a growing number of states, including Colorado, Maryland, Maine, Oregon, and Washington, that have recently passed legislation to fund and support PDABs— organisations that can review drug prices, and in some cases, set a cap on their price through an upper payment limit (UPL).
Earlier this year, two state PDABs—in Colorado and Maryland—completed the first step of deeming certain drugs ‘unaffordable,’ and proposed payment limits, triggering a series of steps that are designed to implement these limits and reduce costs.
State PDABs are still relatively new in the continuum of drug pricing policy. However, as drug wholesalers and some patient organisations push back against certain recommendations, and pharma companies pursue legal challenges, PDABs are approaching a potential inflection point in their efforts to make drugs affordable.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataWhat is a PDAB?
Drug affordability and overall healthcare costs have remained key concerns for voters. In 2025, the Trump administration pursued direct deals with pharma companies for certain obesity drugs, while the prior Biden administration advanced the Inflation Reduction Act (IRA), which for the first time allowed the Centers for Medicare and Medicaid Services (CMS) to negotiate drug prices.
Separate from these national initiatives, individual states are pushing forward their own plans. This, in part, is because federal reforms have not solved the problem, says Dr. Benjamin Rome, assistant professor of Medicine at Brigham and Women’s Hospital in Boston, Massachusetts.
Rome says states have strong reasons to engage on drug pricing; for one, they fund Medicaid programs, and high prescription drug spending is a burden on state budgets. Secondly, states are also responsible for funding health insurance for state employees.
Each state PDAB is unique in its scope and target population—some cover all state residents, while others focus only on Medicaid enrollees—but all aim to make prescription drugs more affordable. Maryland’s PDAB recommendations were initially limited to cover only state-sponsored health plans, but a bill passed this year allowed for its reach to be expanded to all state residents.

How drugs are priced
More than 65% of individuals in the US are covered by private insurance agencies, which classify drugs and determine how a patient gets them in distinct ways through mechanisms such as formulary tiers, and rules like prior authorisation.

Pharmacy benefit managers (PBM) act as middlemen between a pharmaceutical manufacturer and insurance company to negotiate the price for a drug—separate from the publicly known list price or sticker price of a drug—and such confidential deals often involve bundling several drugs and rebates. Each individual patient then pays a copay and coinsurance depending on their insurance plan to receive a prescription drug.
This complexity bleeds down to patients who must navigate changes in the coverage and tiers within plans. Intermill says loss of coverage for her arthritis treatment is a constant concern, noting that if her biologic, which costs approximately $52,000 a year, were no longer covered, she could not sustainably afford it over time.
At the heart of it all, however, is the drug’s list price, and Jane Horvath, an independent health policy expert, says companies are incentivised to keep those high due to market forces or let Wall Street “punish” them.
For example, in November, when Novo Nordisk announced it would lower the price of its weight loss drug Wegovy (semaglutide) by selling directly on the TrumpRx website, the company—valued at $1.07 trillion—saw its stock fall nearly 5%. Eli Lilly, which announced a similar deal, experienced a stock drop of almost 1.4%.
Horvath previously worked at MSD for over a decade and drew on that experience to spearhead some of the early policy proposals around PDABs aimed at addressing rising drug prices.
One of the most controversial proposals involves setting an upper payment limit or UPL. She explains that the intent was never to harm manufacturers. Rather, the goal is to ensure state-licensed bodies—pharmacies, wholesalers, and hospitals—do not purchase a drug above its UPL, which in turn encourages wholesalers to negotiate a differential price with manufacturers for a specific state.
The trade-off on lowering the cost of the product could be lower revenue for a manufacturer, but the goal is that more people can afford their medicine and potentially increase utilisation.
Medicare’s MFP lends a hand
In early October, the Colorado PDAB became the first to consider using the maximum fair price (MFP) negotiated under the IRA with Amgen for its drug Enbrel (etanercept), which is prescribed for rheumatoid arthritis and other autoimmune diseases. After a thorough review, the board decided to choose $583.59 per unit, and approximate annual cost $31,000, and will go into effect in 2027.
An Amgen spokesperson told Pharmaceutical Technology the company will be presenting its argument in Colorado’s federal court and that the board acted “unlawfully” in setting a UPL, adding: “Not only is Colorado’s law unconstitutional, but price controls will also not meaningfully address affordability at the pharmacy counter and will instead create new access barriers for patients.”
In November, the Maryland PDAB also recommended using the MFP as a payment limit for the monthly supply of Boehringer Ingelheim’s Jardiance (empagliflozin)—$197 and AstraZeneca’sFarxiga (dapagliflozin)—$178.50. While both companies did not respond to a request for comment, they submitted detailed documents against any UPL during the Board’s public comment process.
There is an exciting convergence between the state PDABs and the Medicare drug price negotiations, and using the MFP would make the logistics easier, says a US health policy expert. In fact, states cannot set a payment limit for a drug for which a federal agency has already set a payment limit without being sued, adds Horvath.
Inter-state logistics make matters complicated
Nonetheless, enacting distinct policies can be challenging for states given the highly fragmented nature of the US healthcare system. For example, Leah Lindahl, who represents pharmaceutical wholesalers through the Healthcare Distribution Alliance, notes that a warehouse in Colorado is not solely supplying products to that state; it may also distribute them to neighboring states such as Utah. Wholesalers operate on national contracts with manufacturers, and a patchwork of policies across different states, at the same time that federal MFPs are coming into place, put significant strain on the supply chain, explains Lindahl. This can be challenging, because for example, prior to the Drug Quality and Security Act (DQSA) created national standards for licensing of drug wholesale distributors in 2013, each state had different unique licensure requirements, which led to “grey market activity” or wholesalers being licensed heavily, says Lindahl.
It is unclear which party—a manufacturer, drug wholesaler, or pharmacy—would bear any potential cost deficit due to a UPL. According to Lindahl, a UPL would primarily affect pharmacies, which would need sufficient financial overhead. While the product’s reimbursement rate remains unchanged, the sale price would likely be altered due to the UPL.
“I think it is up in the air, kind of who would bear the burden of it, and how it would actually work once operationalised,” says Lindahl. The National Community Pharmacists Association has highlighted similar concerns of pharmacies being forced to “[float] the difference between acquisition costs and government-set amounts” under a proposed UPL or even the negotiated MFPs for the selected drugs.
However, Horvath says that even in the current system, wholesalers can buy a drug at a UPL in a particular state and claim a chargeback from the pharma company. Payers—including the Department of Veterans Affairs, Medicare, Medicaid, and commercial payers—already negotiate different payment rates, so the market should be able to handle the logistics of having a UPL on a handful of drugs in one state, the policy expert says.
Still, outside healthcare, there is precedent for state agencies regulating public utility prices, particularly in cases of monopoly, says Horvath. This model provided a useful reference when developing PDABs, as state utility commissions don’t negotiate with companies, but instead set a payment limit of what can be charged to consumers.
Will states be able to regulate drug prices in the US?
Despite progress in several states, budget and operational challenges have constrained others. In July 2025, New Hampshire dissolved its PDAB, which had been established in 2020.
Lindahl says the various state PDABs need to slow down to study the impact of UPLs more thoroughly and understand the roles of different supply chain stakeholders to avoid unintended consequences.
Looking ahead, the health policy expert says the Maryland and Colorado PDABs are the ones to watch. The Minnesota PDAB, while new, could catch up quickly because of the state’s health data infrastructure, according to Horvath and the expert.
In the coming months, Intermill hopes the Minnesota PDAB will review drugs that have already negotiated MFPs since much of the groundwork is already in place. While her biologic is not on the list, she says any changes could still benefit patients on the margins. She also points out that unlike other products, patients often cannot shop around for drug prices.
“There is a biologic that works for me, and if I don’t take it, I can’t close my fist.”
This story is part of a reporting fellowship sponsored by the Association of Health Care Journalists (AHCJ) and supported by The Commonwealth Fund.




