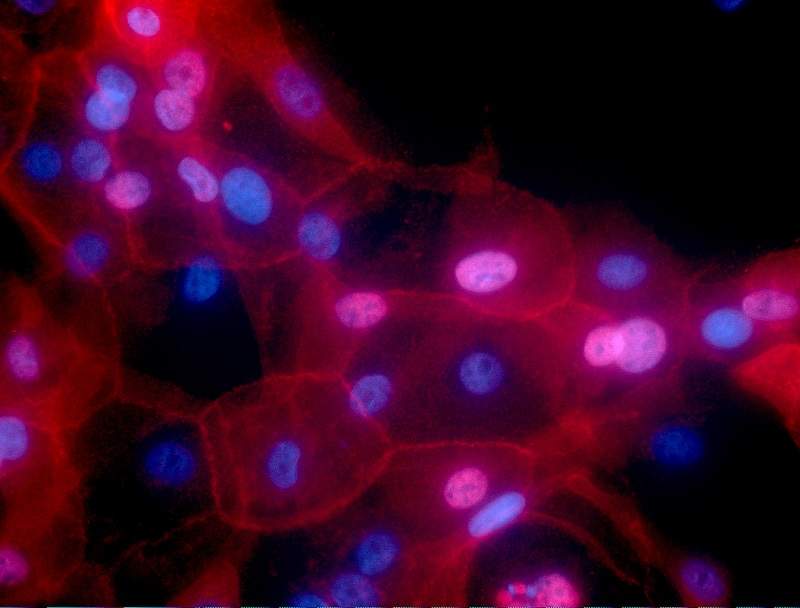
‘Mini tumours’ developed to test cancer drugs
Researchers found a way to test cancer drugs on patient-specific ‘mini tumours’ grown in the lab to help doctors know in advance what drugs will work.
Published in Science and conducted by researchers from the Royal Marsden and the Institute of Cancer Research, the study looked at whether mini-tumours could be used to predict how patients would respond to treatment.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The mini tumours were grown from tissue samples from 71 patients with advanced bowel, stomach or bile duct cancer whose tumours had spread round the body. The tissue samples were then grown inside a gel to create miniature ‘organoids’ with a 96% similarity to the original tumours. Researchers then tested 55 cancer drugs on cells from the samples.
Roche agreed to take over Flatiron Health for $1.9bn
Roche entered a definitive agreement to acquire all shares of Flatiron Health, a US-based healthcare technology and services firm, for a total consideration of $1.9bn.
The deal expands Roche’s current 12.6% equity stake in the firm and is expected to aid in the development and delivery of new innovative medicines for cancer patients.
Flatiron Health carries expertise in oncology-specific, electronic health record (EHR) software and generation of real-world evidence for cancer research.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataDanish researchers created new approach to make QR-coded medicines
Researchers at the University of Copenhagen, Denmark, partnered with Åbo Akademi University in Finland to develop a new manufacturing method for personalised medicines.
The new approach can be used to print medical drugs in QR-coded patterns onto a white edible material. It is expected that the shape of a QR code will facilitate storage of data or information inside the medication, leading to the prevention of incorrect medication or fake products.
University of Copenhagen pharmacy department assistant professor Natalja Genina said: “This technology is promising, because the medical drug can be dosed exactly the way you want it to.
Teva concluded $703m women’s health products deal with CVC Capital
Teva Pharmaceutical completed the divestiture of a portfolio of products under its specialist global women’s health business to CVC Capital Partners for $703m.
Marketed outside the US, the range includes products such as Ovaleap, Zoely, Seasonique, Colpotrophine and Actonel across contraception, fertility, menopause and osteoporosis segments.
This transaction is one of two definitive agreements signed by Teva in September last year to divest the remaining assets of the specialist business for a total consideration of $1.38bn.
Organ-on-a-chip start-up partners with Roche and Takeda
Roche and Takeda announced that they will be using organ-chip technology in their R&D programmes through partnerships with Emulate, the biotech start-up behind organs-on-a-chip.
Emulate and Roche formed a three-year partnership to use organ-chips for testing the efficacy and safety of new antibody therapeutics and combination therapies.
Working in a jointly run lab, researchers hope to use the chips to gain further insight into disease mechanisms and to increase predictability and early detection of biomarkers. The collaboration will initially use lung-chips and brain-chips with the possibility of using other organ-chips.
NIH launched partnership to advance Parkinson’s disease treatment
The National Institutes of Health (NIH) launched a partnership to advance the treatment of Parkinson’s disease.
The project, which is part of the NIH Accelerating Medicines Partnership, will focus on identifying biomarkers (measurable indicators of the severity or presence of a disease) that could be used as biological targets for the development of new treatments for Parkinson’s disease.
“Advancing treatments for Parkinson’s disease is hampered by insufficient understanding of biological networks; drugs aimed at seemingly promising therapeutic targets fail in clinical trials,” said NIH director Dr Francis S. Collins.
UK study found genetic cause behind drug-resistant typhoid strain
Scientists at the Wellcome Sanger Institute, UK, discovered the genetic cause associated with a strain of typhoid that has become resistant to five classes of antibiotics.
The researchers found that the strain, which caused the current typhoid outbreak in Pakistan, has acquired an extra DNA piece to become resistant to various antibiotics.
Typhoid fever is caused by the Salmonella enterica serovar Typhi bacterium, which is highly contagious. The ongoing outbreak of drug-resistant typhoid in Pakistan began in November 2016.
New Zealand researchers began development of natural remedy for diabetes
Researchers from the University of Otago in New Zealand began developing a new natural product extracted from the dahlia plant to potentially prevent diabetes.
Dr Alexander Tups from the university’s Centre for Neuroendocrinology said that the product could be favourable for people with prediabetes, where blood glucose levels are higher than normal but not enough to meet criteria for the disease.
According to Tups, when taken early before the development of the condition, the product has the potential to inhibit progression to diabetes.
AbbVie and Voyager targeted one-time treatment for Alzheimer’s
AbbVie signed an exclusive strategic collaboration and option agreement with gene therapy firm Voyager Therapeutics for the development and commercialisation of new treatments for neurodegenerative diseases, including Alzheimer’s disease.
The firms will develop gene therapies containing vectors to deliver monoclonal antibodies directed against the tau protein, the accumulation of which leads to impaired brain function and neuronal cell loss.
They aim to leverage Voyager’s gene therapy platform to formulate a potential one-time therapy that will minimise tau by delivering an AAV vector antibody, which will encode the genetic instructions to generate anti-tau antibodies inside the brain.
MHRA signed agreement for ‘closer collaboration’ with China
The UK’s Medicines and Healthcare products Regulatory Agency (MHRA) signed a memorandum of understanding (MOU) with the China Food and Drug Administration (CFDA) during a visit to China last week.
The agreement, signed by MHRA chief executive Dr Ian Hudson, pledges new areas of cooperation and the exchange of information from the UK’s Accelerated Access Review on the regulation of medicine sold online.
Both countries also reviewed their existing cooperation, building on an MOU signed in 2014, which focused on the exchange of medical safety information.




