
The Addario Lung Cancer Medical Institute (ALCMI) and the Bonnie J. Addario Lung Cancer Foundation (ALCF) have collaborated with UK-based Scancell to assess SCIB2 cancer vaccine to treat patients with non-small-cell lung cancer (NSCLC).
SCIB2 is Scancell's second ImmunoBody cancer vaccine platform currently under development. It stimulates the immune system to potentially treat and prevent cancer.
ALCMI president and chief operating officer Steven Young said: “This partnership enables us to access an important clinical programme that could also accelerate the development of this groundbreaking immunotherapy technology.”
Scancell has successfully completed a Phase I / II clinical trial with SCIB1 cancer vaccine by using it on patients suffering from melanoma.
See Also:
ALCF chair and ALCMI founder Bonnie J. Addario said: “Combining our two foundations' unique resources will increase patient engagement with the goal to bring new treatment options to NSCLC patients.”
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalDataLung cancer accounts for 27% of all cancer deaths, more than breast, prostate and colon cancer combined, according to the Centers for Disease Control and Prevention.
Along with having the ability to complement existing treatments, SCIB2 can also work in cases where current treatments either do not work or are not available.
SCIB2 stimulates immune responses to specific lung cancer antigens that help the vaccine to support the body in targeting and fighting NSCLC, thereby achieving longer survival rates.
Scancell CEO Richard Goodfellow said: “We have generated preclinical data that suggests that SCIB2 could be the ideal complement to existing and emerging checkpoint inhibitor therapies to treat NSCLC and so provide an effective new potential treatment option for patients with this devastating disease.”
ALCMI aims to support the UK company in the design and development of a Phase I / II clinical trial with SCIB2 cancer vaccine in patients suffering from NSCLC.
The clinical trial is expected to commence next year and will take nearly 18 months to complete.
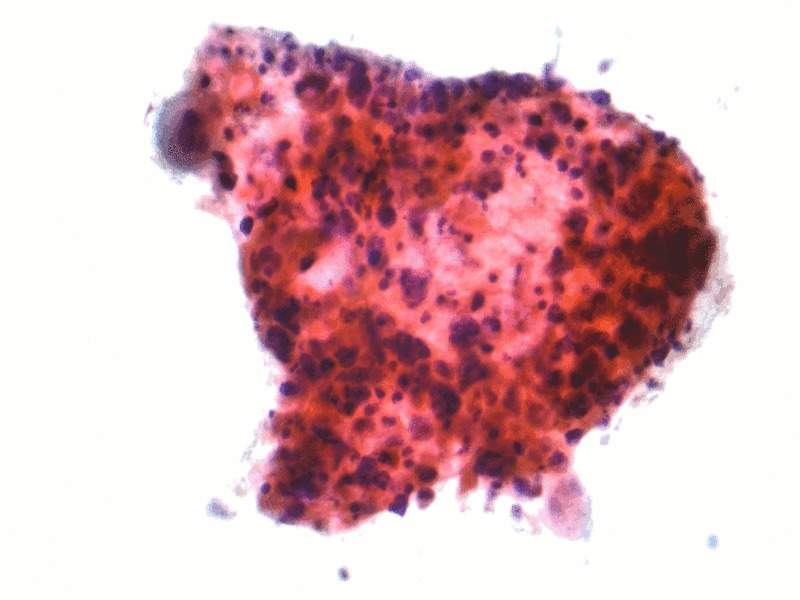
Image: Micrograph of a squamous carcinoma, a type of non-small-cell lung carcinoma. Photo: courtesy of Nephron / Wikipedia.