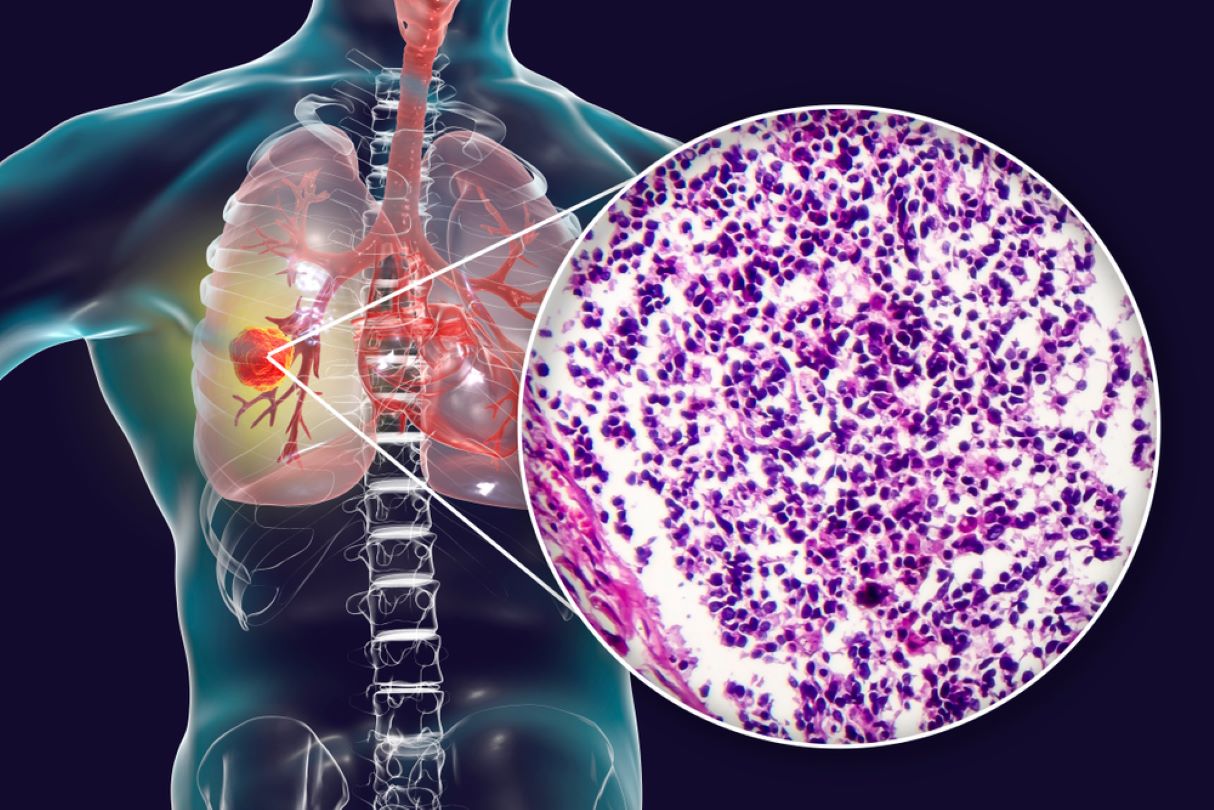
The US Food and Drug Administration (FDA) granted SN Bioscience an orphan drug designation for its small cell lung cancer (SCLC) drug SNB-101, a polymer nanoparticle drug.
The South Korea-headquartered company has received approval to run Phase I clinical trials in the US and South Korea, per a 20 July press release.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The US trial (NCT04640480) is with solid tumours for any type of cancer.
The orphan drug designation programme is an FDA initiative to support the development of new treatments for rare diseases. The classification gives sponsors tax credits from clinical trials, exemption from user fees, and a potential seven years of market exclusivity after approval.
SNB-101 is an intravenously or intratumorally administered drug that acts as a topoisomerase I inhibitor. The therapy causes DNA breaks, inhibiting DNA replication and shutting down the cell cycle to prevent tumour growth. The drug uses the active metabolite (SN-38) of irinotecan as an active pharmaceutical ingredient (API) combined with SN Bioscience’s dual nano-micelle technology. In the press release, the company claims that its therapy has improved the drug resistance and safety of the API compared to other irinotecan drugs.
Pfizer’s Camptosar was the first irinotecan formulation to enter the US market. The FDA approved the drug as a second-line treatment for patients with recurrent or progressive metastatic carcinoma of the colon or the rectum in 1996.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataSince then, several other companies have tested the limits of the API. Ipsen’s irinotecan hydrochloride drug, Onivyde, is also being developed for SCLC and is currently in a Phase III (NCT03088813) clinical trial. The therapy has already received approval for its combination with fluorouracil (5-FU) and leucovorin (LV) in several markets in the US, Europe, and Asia. However, a Phase III trial of the drug did not meet its primary endpoint of overall survival versus the comparator drug in August 2022, per a company release.
According to the American Cancer Society, 10% to 15% of lung cancers are SCLCs. In this condition, malignant (cancer) cells form in the lung tissue. Risk factors for the disease include genetic history, smoking and exposure to radiation.




