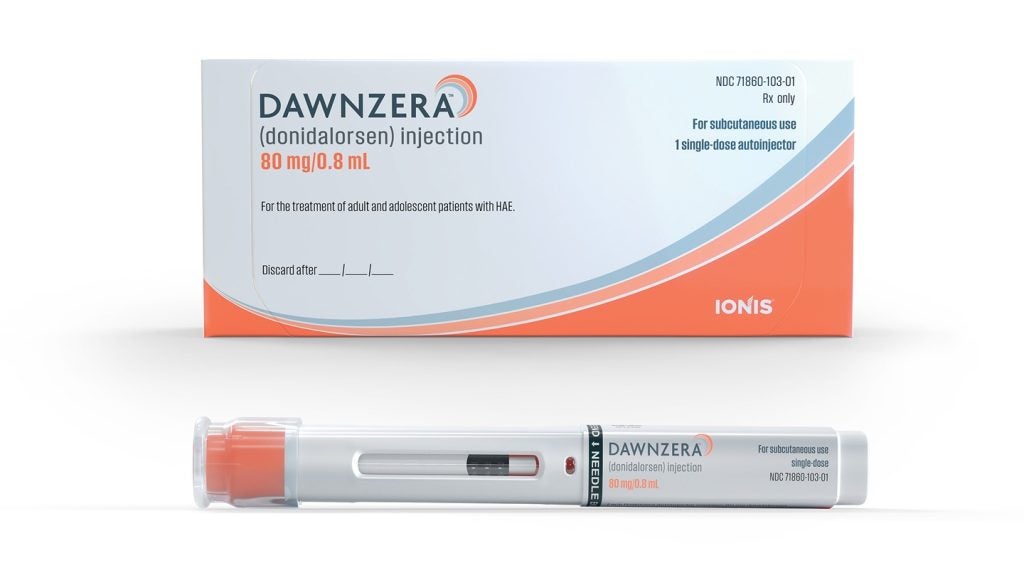
The US Food and Drug Administration (FDA) has approved Ionis Pharmaceuticals’ RNA-targeted prophylactic treatment, Dawnzera (donidalorsen), for use in preventing hereditary angioedema (HAE) attacks in those aged 12 years and above.
Dawnzera is designed to inhibit plasma prekallikrein, a significant activator of the inflammatory mediators that cause acute HAE attacks.
This 80mg dose of the medicine is self-administered through a subcutaneous autoinjector, with patients having the option to choose between dosing every four or eight weeks.
The agency’s approval was influenced by the positive outcomes from the Phase III OASIS-HAE trial.
The placebo-controlled, randomised, global, multicentre, double-blind trial's primary endpoint was achieved, with an 81% reduction in the monthly rate of HAE attacks for those treated with Dawnzera every four weeks, as compared to placebo, over 24 weeks.
This reduction rose to 87% from the second dose, which was a secondary endpoint. The frequency of moderate-to-severe HAE attacks was also reduced by 90% from the second dose onwards during the same timeframe.
Further evidence of Dawnzera's performance comes from the OASISplus open-label extension trial, where the eight-week dosing schedule demonstrated a similar impact to the four-week regimen over time.
After one year, a 94% decrease in the total mean attack rate from baseline was observed across both dosing groups.
HAE is an uncommon genetic condition, characterised by recurrent, severe swelling in various body parts.
Ionis Pharmaceuticals CEO Brett Monia stated: “Dawnzera represents a significant advance for people living with HAE who need improved treatment options.
“With strong and durable efficacy, convenient administration and the longest dosing option available, we believe Dawnzera will be the prophylactic treatment of choice for many people living with HAE.”
In March 2025, Ionis entered a licensing agreement with Sobi. This agreement provided Sobi with the exclusive rights to commercialise olezarsen for the treatment of familial chylomicronaemia syndrome and severely elevated triglycerides.