Quality and Privacy Statement
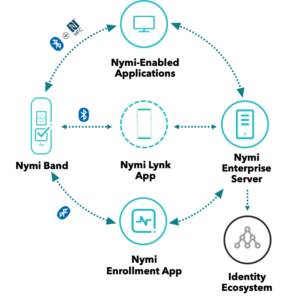
Nymi has implemented a Quality Management System (QMS) that aligns with our customer requirements and guides the development of products and solutions. Nymi develops products and solutions with high traceability to ensure data integrity and security is maintained with the use of our Safe, Secure and Simple Workplace Wearable.
To support our customer needs, we tailor our solutions to enable seamless integration and a structured qualification and validation approach, which is in line with regulatory requirements and GAMP5 recommendations. Additional documentation and guides are provided to support implementation and system validation.
Privacy statement
In Principle
People should not have to sacrifice their privacy in order to use and benefit from technology.
This is the basis of “Privacy by Design” in its most basic concept, and the design principles we uphold. Any amount of data can unintentionally reveal sensitive personal identifiable information (PII), which can land in the wrong hands. The principles of Privacy by Design provide a framework for how technology should be approached to reduce this risk. This means control measures for data privacy are foundational in the design, which minimizes the amount of personal data that is processed and retained.
In Practice
Nymi has been practising Privacy by Design since the inception of the company.
The very nature of a connected workforce requires technology to process some amount of PII, but our approach reduces risk by prioritizing security and privacy beginning from the design of our platform and wearable, to its actual use in everyday life.
Most importantly, we minimize the storage of PII to the least amount required to deliver a workplace solution that increases health and safety, security, and productivity for its users. The result is a powerful technology that is balanced towards the end user’s interests and protects their right to privacy and autonomy at all stages.