Cellares, along with Mitsui Fudosan, is developing a new integrated development and manufacturing organisation (IDMO) smart factory in Kashiwanoha Smart City, Chiba, Japan.
The new facility will handle clinical and commercial-scale manufacturing of cell therapies for cancer immunotherapies, including CAR-T, and is being built by Cellares Japan, a wholly owned subsidiary of Cellares.
Construction of the facility commenced with the groundbreaking ceremony in November 2025, and operations are scheduled to begin in 2027.
The new facility will enable cell therapies to be produced domestically, which will enable Cellares to streamline cold chain logistics and lower overall costs.
The plant will employ around 350 people and forms part of the company’s plan to establish a global IDMO smart factory network across Asia, the US and Europe.
Location
The Cellares IDMO smart factory will be situated within Mitsui Link Lab in Kashiwa City, approximately 30km north-east of central Tokyo in Chiba Prefecture, Japan.
Kashiwa City, in the Tokyo metropolitan area, has been designated by the Cabinet Office as one of eight bio-innovation hubs under the Greater Tokyo BioCommunity initiative.
Cellares IDMO smart factory details
The Cellares IDMO smart factory will be a four-storey, steel-framed facility with a site area of 10,263.43m2 (110,474.6ft2) and a total floor area of 16,791.43m².
The facility will include areas for cell therapy manufacturing, quality control, supply management, administration and mechanical systems.
It will be Cellares’ first automated facility in Asia, producing ten-times more patient doses, cutting operational costs and cleanroom requirements by half.
By leveraging fully automated workflows and standardised platforms, technology transfers from the facility and Cellares sites in other regions are expected to be seamless, fast and driven by software.
Technology details
The factory will use Cellares’ Cell Shuttle™ and Cell Q™ platforms to automate cell therapy production and quality control, with integrated systems intended to minimise downstream bottlenecks.
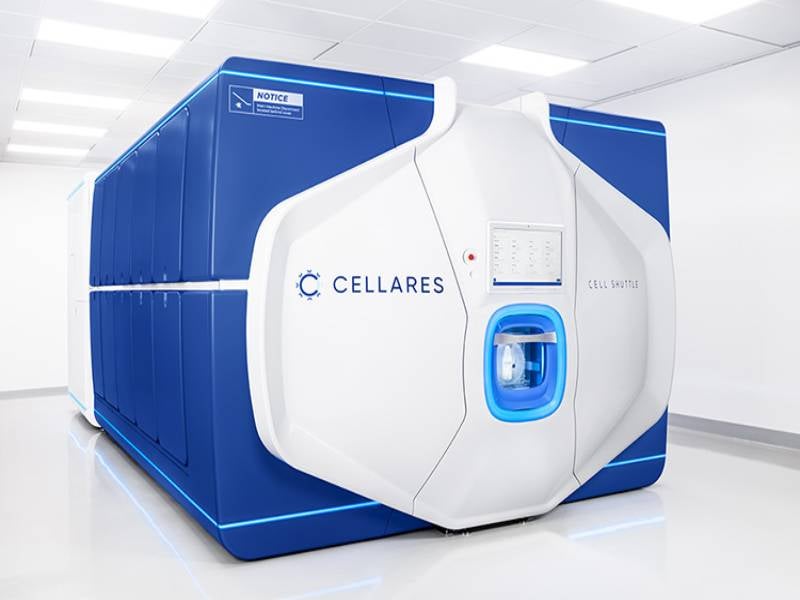
Cellares’ Cell Shuttle is an automated, high-throughput platform for cell therapy manufacturing. It is designed to be a flexible and scalable platform that delivers end-to-end automation.
More than 30 Cell Shuttle units will be installed in the plant, with the capacity to supply treatment for up to 75,000 patients a year.
Cell Q is an automated quality control (QC) workcell for cell therapy manufacturing and can support QC assays for up to 6,000 batches a year with a pre-qualified set of commonly used assays.
Together, Cell Q and Cell Shuttle can handle 16 batches in parallel and are designed to reduce process failures by 75%.
The technology can cut batch costs by as much as 50% and removes the production constraints commonly seen with traditional contract development and manufacturing organisations that rely on manual operations.
In addition, the Smart Factory will be fully integrated and optimised to prevent bottlenecks further downstream.
Contractors involved
The Japan External Trade Organisation’s Invest Japan Business Support Center provided consultation services on incorporation, taxation and labour, sector-specific issues, municipal subsidy support, PR support and introductions to a real estate service provider.
CBRE, in collaboration with Turner & Townsend, provided strategic office advisory services to Cellares during the office leasing phase, the selection of Mitsui Fudosan as a partner and the project location.
Hitachi Plant Services, working with Turner & Townsend, will deliver facility engineering and build infrastructure capable of supporting Cellares’ automation systems.
Turner & Townsend will oversee the full project life cycle from design and construction through to validation and handover, with responsibility for schedule, cost and quality control.
Japan-based Daiwa is also involved in the design and construction of the facility.
Marketing commentary on Cellares
As an IDMO, Cellares applies an Industry 4.0 model to large-scale manufacturing of cell-based therapies. It develops and operates integrated technologies for cell therapy manufacturing to increase access to these treatments.
The company is headquartered in south San Francisco, California, and has opened its first commercial-scale IDMO smart factory in Bridgewater, New Jersey.