GE Healthcare is building a manufacturing facility for cell processing kits in Grens, Switzerland.
The plant, announced in January 2020, is expected to be fully operational in 2022.
It will be primarily used to produce single-use kits, serving as an integrated complex for the company’s research, engineering and service teams. GE Healthcare intends to use its operations in the country to meet the demands of cell and gene therapy customers.
The facility will also expand the firm’s cell processing kits production capacity, expected to employ up to 200 personnel.
GE Healthcare’s manufacturing facility location
Located in Signy Park in Grens, Switzerland, the facility will cover an area of 7,360m².
The site is situated 300m away from the Nyon exit of A1 motorway and 18 minutes away from the Geneva international airport.
Signy Park will spread over an area of 73,000m² and will house offices and school campus.
Details of GE Healthcare’s manufacturing facility
The facility will produce single-use kits for the Sepax and Sefia cell processing systems offered by GE Healthcare Life Sciences.
A Centre of Excellence with a cell and gene therapy manufacturing capability will be built at the site, providing training for the company’s European customers and undertaking educational programmes. The manufacturing facility complex will also support a research and development team.
The Swiss site will allow GE Healthcare to pursue the development of advanced products and cater to anticipated global demand in the cell and gene therapies market.
It will also help the company to attract the best talents in the region to boost its research and development efforts.
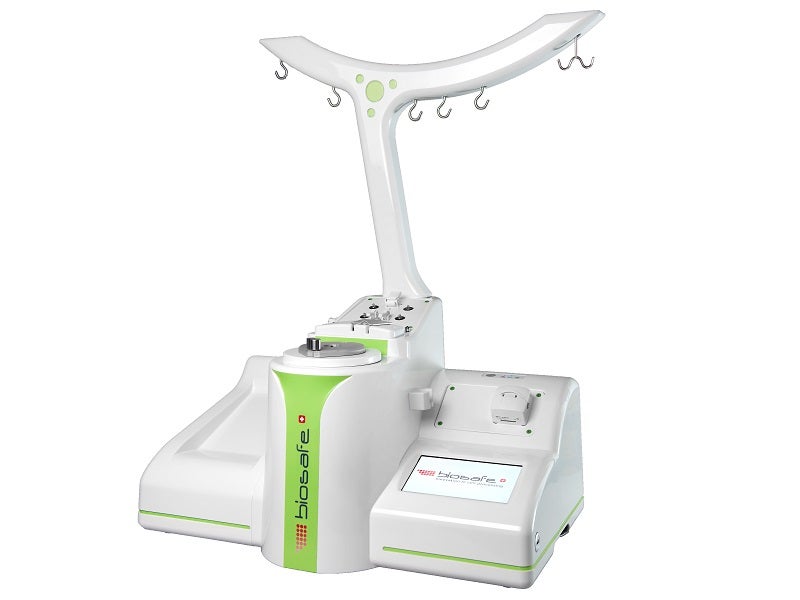
GE Healthcare cell processing systems
Sefia cell processing system is a multi-functional platform by GE Healthcare’s Biosafe Group in September 2016, which supports cell processing during the manufacturing of cell therapy products.
It is highly flexible and functionally-closed laboratory instrument used to simplify processing steps such as isolation, washing, concentration, harvesting and final formulation in cell therapy manufacturing process.
Sefia will allow customers to isolate cells from up to 800ml of apheresis products, allowing users to select steps and run required operations. It will also provide the capability to harvest up to 10l expanded cells using continuous-flow technology. Users can combine multiple steps, running them in sequence automatically. The functionally-closed approach can help companies avoid the risk of contamination.
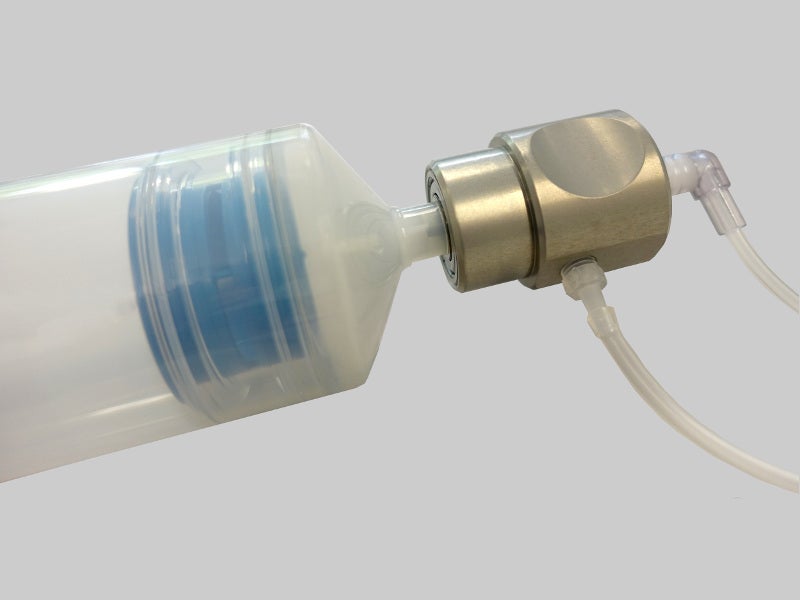
Sepax CPro cell processing system is a functionally-closed system that enables the user to automate the steps involved in the workflow of cell processing.
In upstream processing, users can concentrate, purify, isolate and transduce the required cells. The instrument is for subsequent use along with appropriate protocol software and kit in downstream to harvest, wash, re-suspend and divide the products.
Both systems include a combination of hardware, optimised protocol software and a dedicated kit for the processing of cells.
PremierCell or FlexCell Sefia protocol software can be used with Sefia S-2000 instrument, as well as alongside PeriCell C-Pro, PlateletFree C-Pro, CultureWash C-Pro, SpinOculation C-Pro and Dilution C-Pro, among other Sepax C-Pro software protocols.
The SpinOculation C-Pro protocol software released in December 2019 streamlines process validation and decreases variability encountered in the cell transduction step.
Single-use kits of Sepax C-Pro cell processing system include CT-49.1, CT-60.1 and CT-90.1.
Both systems are CE certified and good automated manufacturing practice (GAMP)-compliant.
Contractors involved
Dutch-Swiss developer Nemaco manages the construction and design of GE Healthcare’s manufacturing faculty.
Marketing commentary on GE Healthcare
GE Healthcare is an American multinational medical technology and life sciences firm that provides a range of products and services for medical research industries.
Headquartered in Chicago, Illinois, US, the company develops medical technologies, digital systems, tools and data analytics to serve the needs of professionals in the healthcare industry.
The Life Sciences division of the company focuses on accelerating the discovery, manufacturing and use of therapies and precision diagnostics. It provides tools and technologies to assist researchers and companies in cell and gene therapy development and commercialisation, as well as in genomics and cellular research.
GE Healthcare offers design solutions tailored to meet the requirements of customers, licensing agreements and contract manufacturing services concerning reagent preparation and kit manufacturing. The company provides a range of services including preventive maintenance of equipment, asset management over the life cycle, validation services and bioprocess development services.
GE has been operating in the healthcare industry for more than 100 years and employs approximately 50,000 employees in more than 100 countries globally.