In August 2013, NanoViricides began building a clinical-scale current good manufacturing practice (cGMP) production plant at the industrial-commercial zone on the Route 8 Corridor in Shelton, Connecticut.
The facility was officially opened in July 2014 and is engaged in the production of active pharmaceutical ingredients (APIs). NanoViricides invested $20m in the construction of the facility.
Inno-Haven, a privately owned company controlled by the president of NanoViricides, was contracted to build the cGMP and research and development (R&D) project.
In August 2011, a 4.2-acre facility with an 18,000ft² building located at the industrial-commercial zone in Shelton was bought for the project.
In 2013, NanoViricides entered a contract with Inno-Haven to lease the cGMP manufacturing facilities until the building was fully renovated. The contract agreement included the option of future purchase. NanoViricides purchased the cGMP production facility from Inno-Haven in January 2015, instead of leasing.
Details of the NanoViricides cGMP facility in Connecticut
The cGMP facility will be built by renovating the existing 18,000ft² building. New research labs, offices and a cGMP pharmaceutical manufacturing suite with cleanrooms will be built at the site.
The facility will be used for the production of NanoViricides’ drug substances for clinical trials using cGMP processes. The substances are supplied to a third party for final processing and labelling.
They will also facilitate the necessary investigational new drug-enabling studies for anti-influenza drug candidates for future human clinical studies.
Construction of the cGMP facility and contractors involved in the project
Ground was broken for the plant’s construction in August 2013. Demolition of the previous facility at Shelton was completed in July 2013.
The facility’s design, engineering and architecture contract was awarded to Id3A in January 2013.
The contractual scope included providing the overall facility architecture, with the integration of a separately constructed cleanroom suite for cGMP production. The design was completed by the end of June 2013.
MPH Engineering was appointed as the project manager and engineering consultant for the construction of the facility. Bristol Myers Squibb was selected to design the project.
Technology at the API production facility
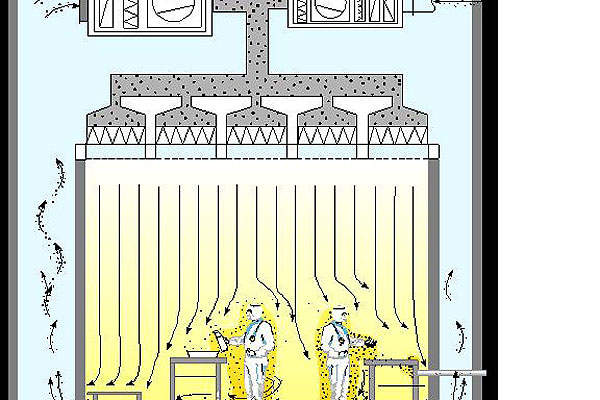
The facility features a cleanroom suite, the design and development contract for which was awarded to AES Clean Technology (AES) in January 2013.
AES’s modular cleanroom technology is designed to provide a quicker, cleaner and more qualitative and repeatable performance.
The contractual scope includes the supply of Class 100 laminar hoods, chemical fume hoods and Class 1,000 and Class 10,000 work areas, as well as entry airlock and egress systems.
Drugs produced at the NanoViricides plant in Connecticut
The facility is engaged in manufacturing drugs against a number of viral diseases, such as H1N1 swine flu, H5N1 bird flu, seasonal influenza, HIV, oral and genital herpes.
It also produces drugs against viral eye diseases, such as herpes keratitis, as well as Hepatitis C, rabies, dengue fever and ebola virus.
The plant’s product portfolio includes FluCide, DengueCide and anti-flu drug candidates NV-INF-1 and NV-INF-2, in oral and injectable versions.
Marketing commentary on NanoViricides
NanoViricides is a development-stage, nano-biopharmaceutical company based in Shelton, Connecticut.
The company discovers, develops and commercialises therapeutics for viral infections, producing a class of drugs designed specifically to attack and eradicate their surrounding virus particles.
NanoViricides has reached agreements with Public Health England (PHE) and the Lovelace Respiratory Research Institute (LRRI) in the US to carry out further testing on its FluCide drug candidate against additional influenza viruses, including H7N9.