Clinical-stage biotechnology company Vaxxas opened a new biomedical manufacturing facility in Brisbane, Australia, in June 2023.
The new facility is designed to support the scale-up of Vaxxas’ needle-free vaccine technology platform named high-density microarray patch (HD-MAP) for late-stage clinical trials and first commercial products.
The facility is expected to produce approximately 300 million vaccine doses a year once fully operational and also serve as the global headquarters of Vaxxas.
The first commercial products of the company are expected to be distributed globally from the facility in the next three to five years.
Location of Vaxxas’ biomedical manufacturing plant
Vaxxas biomedical manufacturing facility is located at the Northshore Priority Development Area in Northshore Hamilton in Brisbane, Queensland.
The state-of-the-art facility was developed through the refurbishment of an existing warehouse at Northshore Hamilton.
Vaxxas’ biomedical manufacturing facility details
Spanning across 5,500m² (60,000ft²), the new facility includes two independent Good Manufacturing Practice (GMP) qualified aseptic cleanrooms, a device assembly cleanroom, and medical device manufacturing space. It also includes supporting infrastructure such as laboratories and office space.
Specialised manufacturing infrastructure is due to be installed at the facility in 2024, which will enable the scale-up of its vaccine patch production capacity.
The manufacturing at the facility will meet the standard requirements of the Australian Therapeutic Goods Administration, the European Medicines Agency, the US Food and Drug Administration, and other global health regulators.
HD-MAP technology details
Vaxxas’ core proprietary HD-MAP technology was initially developed at The University of Queensland.
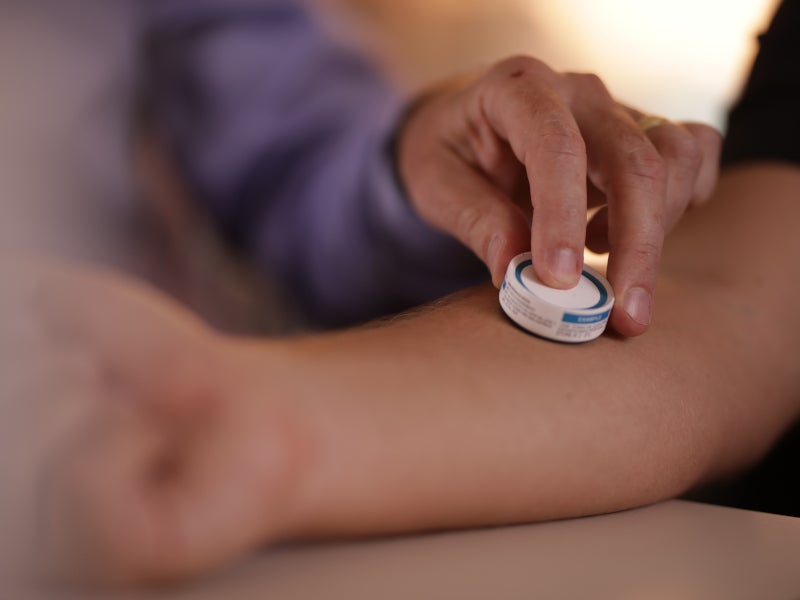
HD-MAP is a needle-free delivery system designed to enhance the efficacy and efficiency of vaccines by targeting the skin’s immune cells and optimising the body’s immune reaction. It consists of a small, flat patch with an array of thousands of tiny projections called microprojections, each measuring less than half a millimetre long.
When the patch is applied to the skin, the microprojections coated with vaccine antigens painlessly penetrate the outermost layer of the skin, targeting the rich network of immune cells just beneath the surface.
The HD-MAP technology eliminates the need for needles and syringes, addressing the challenges associated with traditional vaccine administration.
Needle-based injections can cause discomfort, require skilled personnel for administration, and generate medical waste. In contrast, HD-MAP technology offers a painless, user-friendly and self-administration alternative, making vaccinations more accessible and reducing the risk of accidental needlestick injuries.
In addition, the high volume and low manufacturing cost of the microarray patch enable its worldwide distribution in a more cost-effective way than traditional vaccines.
Furthermore, the transportation of vaccines in rural and remote communities becomes easier as vaccine patches can be stored at high temperatures such as 40°C.
The HD-MAP technology is currently being evaluated in six clinical programmes with a number of vaccines.
Funding for Vaxxas’ plant and HD-MAP technology
Vaxxas received a A$4.4m ($3.3m) grant in July 2021 under the first round of the Australian Federal Government’s Modern Manufacturing Initiative (MMI) to support the scale-up of the HD-MAP technology and development of the new facility.
Vaxxas received a grant of A$8.2m ($5.5m) in September 2022 in the second round of the MMI.
Contractors involved
Australia-based construction company Hansen Yuncken was awarded the contract to refurbish and transform the existing warehouse into a biomedical facility.
Marketing commentary on Vaxxas
Vaxxas was founded in 2011 as a spin-off from the University of Queensland. It specialises in the development of innovative vaccine delivery technologies.
The company was founded with initial equity financing led by OneVentures, a venture capital firm, with co-investors Brandon Capital Partners and HealthCare Ventures.
Vaxxas’ partners include the World Health Organisation (WHO), the Bill and Melinda Gates Foundation, Coalition for Epidemic Preparedness Innovations (CEPI), the Australian Federal and Queensland State Governments, and the US Government.
Vaxxas has also signed a collaboration and licence agreement with pharmaceutical company Merck & Co (MSD) to utilise the proprietary HD-MAP platform for an undisclosed vaccine candidate from its vaccine pipeline.
Vaxxas has also formed an alliance with pharmaceutical process engineering company Harro Höfliger to develop a high-throughput, aseptic manufacturing line to produce vaccines based on the HD-MAP technology.