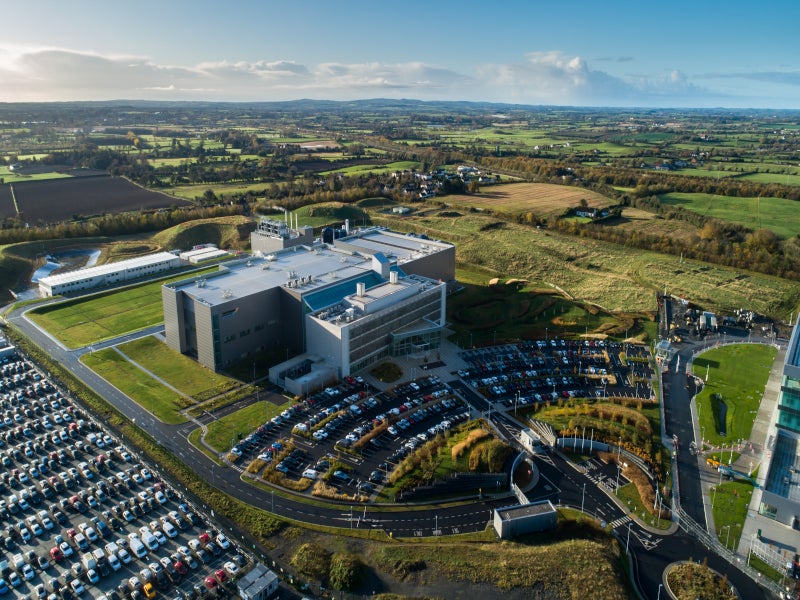
China-based development and manufacturing company WuXi Biologics’ biologics manufacturing facility in Dundalk, Republic of Ireland, was built with an investment of €325m ($394m).
The construction of the state-of-the-art manufacturing facility began in December 2018, and operations began in December 2021.
The facility is among the first and the largest greenfield pharmaceutical projects by a Chinese company in Ireland. It manufactures monoclonal antibodies and recombinant proteins.
It has enabled WuXi to expand its commercial manufacturing capacities and strengthen its global integrated solution platform.
The project created more than 2,000 jobs during construction and over 500 highly skilled local jobs for Ireland’s North-East region. The site currently employs more than 7,000 personnel.
In August 2025, the European Medicines Agency (EMA) approved WuXi Biologics’ Dundalk site in Ireland to manufacture a client’s novel biologic for commercial supply.
The approval comes after the EMA and US Food and Drug Administration (FDA) had previously authorised commercial manufacturing of the same product at other WuXi Biologics facilities since 2023.
With the decision, the Dundalk facility is now cleared for commercial production for the first time, following its full Good Manufacturing Practice (GMP) authorisation from Ireland’s Health Products Regulatory Authority (HPRA) in 2024.
WuXi Biologics facility location
The new manufacturing facility is developed on a 26ha site in Mullagharlin between Dublin and Belfast.
The site lies close to towns such as Armagh, Balbriggan, Cavan, Dundalk, Drogheda, Monaghan, Newry and Swords.
It offers easy access to three international airports, an intercity rail service, motorway connectivity, and deep seaports.
Dundalk was chosen as the site due to the Industrial Development Authority’s (IDA) endorsement of the regional and property strategy.
WuXi Biologics’ biologics manufacturing facility details
The 43,432m² (467,492.3ft²) greenfield facility consists of seven buildings, including a three-storey manufacturing facility with a floor area of 21,020m², an 11,051m² laboratory and administrative building and a two-storey central utility building (CUB) with a floor area of 2,230m².
The facility also includes electrical and security buildings, as well as a wastewater treatment plant.
It features two cell culture manufacturing areas, with the first one equipped with six 1,000l single-use bioreactors for perfusion (continuous) processing.
The second cell culture manufacturing area (MFG7) is equipped with 12 single-use bioreactors with a capacity of 4,000 litres each, offering a total capacity of 48,000 litres.
The area is designed for fed-batch, concentrated fed-batch, and intensified fed-batch techniques.
The plant is also equipped with seven eco-steam washers and eight high-performance sterilisers.
It is designed to support large-scale commercial manufacturing with disposable bioreactors, as well as next-generation continuous bioprocessing manufacturing technology.
The first 16,000l manufacturing run at the MFG7 facility was completed by combining four 4,000-litre single-use bioreactors in January 2024.
The run opened the door for large-scale commercial manufacturing projects at the site in Ireland.
WuXi Biologics facility design details
The production building, the first structure erected at the facility, has three floors and two intermediate mezzanines.
It features a steel-braced frame designed with a 9m × 9m column grid pattern. Simultaneously, the CUB, measuring 42m × 27m, was constructed nearby.
The braced structure is independent but connected to its neighbour by an atrium. The 25m-high atrium connects the laboratories and administration facilities with the industrial buildings.
The atrium features a sculptural staircase that provides access to all the floors in the building, which is clad with dark grey and silver-grey metal insulated panels and double-glazed aluminium windows.
One end of the production building connects to the warehouse via a two-storey link, though they are structurally independent and separated by columns and a movement joint.
The warehouse measures 80m × 30m. Running parallel to the production building, the four-storey laboratory and administration building also measures 80m × 30m.
The laboratories are designed with an open-plan concept, collaborative furnishings, and a clean aesthetic.
The interiors of the facility feature a bio-resin terrazzo floor. A woven vinyl flooring was installed in the offices while high-density Bakelite-core floorboards were installed in the maintenance rooms.
The site achieved a weather-tight seal for its main facility in December 2019, utilising 5,500 tonnes of structural steel and 8,000m² of wall cladding.
Unlike most similar facilities, the clean rooms at the site feature extensive windows facing the outer corridor, allowing natural light to benefit workers and enabling visitors to view manufacturing processes without entering the area, thereby reducing contamination risk.
The manufacturing lines are highly automated to minimise the need for personnel to enter clean areas, further reducing cross-contamination and bioburden.
The design ensures an efficient GMP environment while maintaining a comfortable, energy-efficient workspace.
Sustainability features
The facility has adopted sustainability concepts in the site construction and operations, such as reduced use of energy and recycling resources.
The site is completely powered by 100% renewable energy sources, achieving a 1.7GWh decrease in electricity usage and a 3.4GWh reduction in natural gas consumption.
It has consistently implemented various waste reduction initiatives to mitigate its environmental impact.
WuXi Biologics facility certifications and awards
The Dundalk manufacturing facility received its first GMP certificate from the Irish Health Products Regulatory Authority within nine months of the start of operations.
In May 2023, the International Society for Pharmaceutical Engineering recognised the production facility in Dundalk, Ireland, with the 2023 Facility of the Year Award in the operations category. It was also honoured with the 2023 Irish Construction Excellence Award.
In March 2024, the Ireland facility received three International Organisation for Standardisation (ISO) certifications: ISO 50001 – Energy Management Systems, ISO 14001 – Environmental Management Systems, and ISO 45001 – Occupational Health and Safety Management Systems.
It has also implemented comprehensive Environment, Health & Safety programmes in line with ISO 45001 to ensure a safe and healthy workplace.
Financing for the WuXi Biologics facility
The Irish Government supported the project through the IDA and Ireland Strategic Investment Fund, a sovereign development fund managed by the National Treasury Management Agency.
Contractors involved
Scott Tallon Walker Architects (STW), an architecture company, designed the facility, ensuring IDA general planning permission and WuXi’s global design excellence.
Engineering, construction and consulting company Jacobs provided construction management services for the facility development using the Integrated Project Delivery methodology.
IPS, an engineering, procurement and construction management company, provided detailed design services.
Lawler Consulting, a building services and sustainability consulting services provider, was responsible for the mechanical and electrical design services.
GDCL Consulting Engineers, a civil and structural engineering services supplier, was responsible for the civil and structural engineering design.
Construction company PJ Hegarty was awarded the contract to construct the facility while Ardmac, a construction specialist, was contracted to undertake cleanroom installation.
Mercury, a construction solutions company, was contracted for design completion, mechanical and electrical works, and equipment installation while Wilec, a fire, safety, and security systems installation services provider, was awarded the life safety and security systems contract.
ABEC, a biopharmaceutical manufacturing systems and solutions supplier, provided 12 bioreactors for the facility.
Fedegari Autoclavi, an equipment and systems supplier, supplied 15 high-performance process machines, including washers and autoclaves.
Cost control services were provided by Linesight, a construction consultant, while Suir Engineering, an engineering company, handled the electrical and instrumentation package.
Collen Construction provided fit-out services for the headquarters building of the facility.
Other contractors involved in the project are Kiernan Structural Steel, LPI Group, Capcon Engineering, DCT, CORE Solutions, Casala and Bene Ireland.