Triple negative breast cancer (TNBC) is characterised by a lack of clinically significant expression levels of oestrogen receptor, progesterone receptor and human epidermal growth factor receptor 2 (HER2). This type of cancer therefore tends to have a worse prognosis and fewer treatment options than other breast cancers. Nevertheless, immune checkpoint inhibitors have substantially impacted treatment outcomes for patients with TNBC and have led to a paradigm shift of testing triple-negative tumours for programmed death ligand 1 (PD-L1) when considering chemoimmunotherapy for metastatic disease. Consequently, most of the marketed and pipeline drugs for the treatment of TNBC target programmed cell death protein 1 (PD-1).
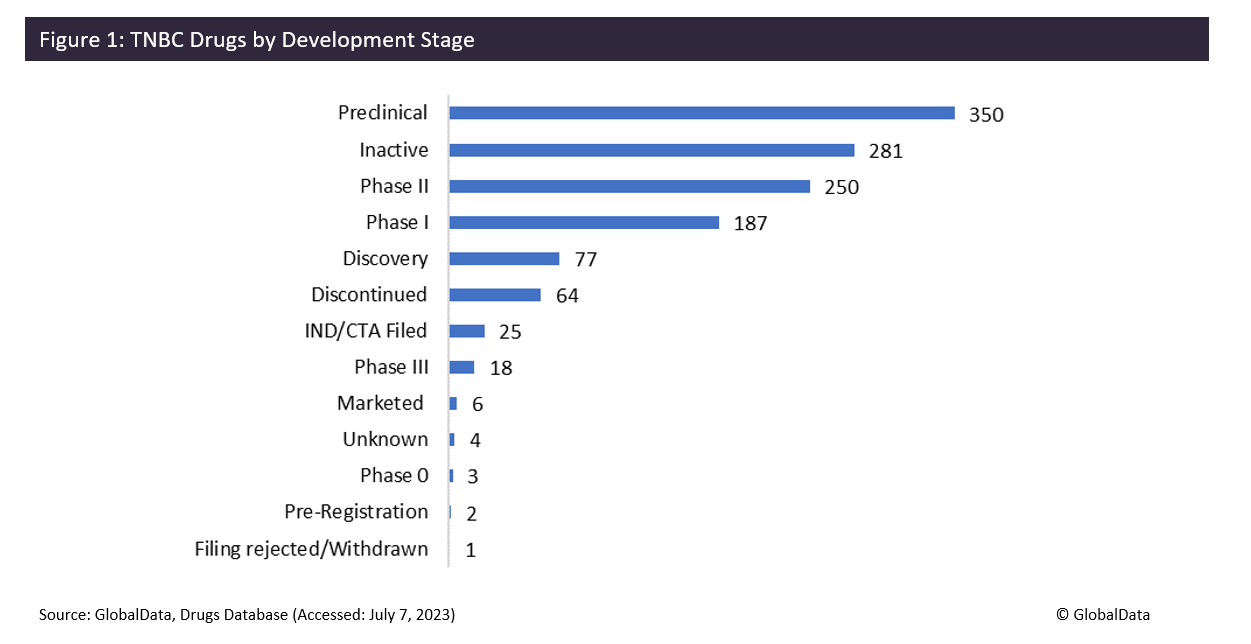
TNBC has a lack of treatment options compared to other breast cancer subtypes, with only six drugs currently on the market. TNBC tends to grow and spread faster than other types of invasive breast cancer, so is traditionally considered the most difficult breast cancer type to treat, and patient outcomes have been dismal. This could explain why such a large proportion of drugs are failing to make it to Phase III trials – proportionally fewer TNBC drugs (7%) progress from Phase II to III than both HER2- (38%) and HER2+ (55%) breast cancers. However, there are 350 drugs in preclinical development for TNBC, highlighting that there is some recognition of the huge demand for new therapies.
Merck & Co’s immune checkpoint inhibitor Keytruda (pembrolizumab) is a monoclonal antibody (mAb) that antagonises PD-1, releasing PD-1 pathway-mediated inhibition of the immune response and enabling T cells to attack cancer cells.
This drug was approved in July 2021 for high-risk, early-stage TNBC in combination with chemotherapy as a neoadjuvant treatment. Roche’s Tecentriq (atezolizumab) is another immune checkpoint inhibitor that is an anti-PD-L1 mAb, boosting the immune system’s capacity to stop cancer growth. However, in June 2021, after several years on the market, Roche withdrew its application to extend the use of atezolizumab to the treatment of patients with early or locally advanced TNBC in Europe. This came after confirmatory trials did not indicate a clinical benefit to treatment, providing just one example of the difficulty of getting new drugs to the TNBC market.
Daiichi Sankyo’s Enhertu (trastuzumab deruxtecan) was approved by the FDA in August 2022 for the treatment of patients with unresectable or metastatic HER2-low breast cancer. HER2-low breast cancer is a newly defined subset of HER2- breast cancer that has some HER2 proteins on the cell surface, but not enough to be classified as HER2+. The approval was based on the DESTINY-Breast04 clinical trial, the results of which showed improvement in both progression-free survival and overall survival in people with HER2-low breast cancer. Therefore, Enhertu is likely to positively impact the treatment of TNBC as it provides a new treatment option to TNBC patients who express HER2 at low levels.
One pipeline drug that has a similar mechanism to pembrolizumab is Shanghai Junshi Biomedical Technology’s Tuoyi (toripalimab), an IgG4K mAb that targets PD-1. The TORCHLIGHT clinical trial is a Phase III, double-blind study in which patients were randomised to receive toripalimab against a placebo, in combination with nab-paclitaxel for newly metastatic TNBC. Early data suggested that toripalimab may improve overall survival, proving to be a very interesting new drug. However, these results are not statistically significant at this time.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThis means that competing PD-1 inhibitors are also unlikely to make an impact in the metastatic TNBC setting, as their results will not be substantially different from toripalimab. AstraZeneca’s Imfinzi (durvalumab) is another pipeline drug that acts as a PD-L1 blocking antibody and is currently being investigated in a Phase II study to investigate its addition to paclitaxel chemotherapy in TNBC. The results presented from this trial in June 2023 showed that the scores for immune cell types representing macrophages and neutrophils increased significantly during treatment, whereas scores for B cells, T cells and Th1 cells decreased regardless of the treatment group. This suggests that durvalumab may not be massively effective at boosting the immune system’s response to a tumour.
Overall, it seems clear that there is a significant need for novel therapeutics to treat TNBC, but finding a drug with clinical benefits is proving difficult due to TNBC’s low response to therapeutics and highly invasive nature. As TNBC is responsible for roughly 15% of breast cancers, further research and clinical advancement in this area is crucial in order to expand survival rates from this aggressive cancer.