US-based pharmaceutical company Pfizer began building a biologics clinical manufacturing facility at its existing manufacturing facility in Andover, Massachusetts, in June 2016. The new facility will be used for producing high-quality, complex biologics and vaccines and is expected to come online by January 2019.
Estimated to cost $200m, the project will create 200 construction jobs and 75 clinical manufacturing roles. It will have clinical manufacturing capacities designed to provide the lowest cost and greatest flexibility for efficiently driving new treatments.
Details of the Pfizer manufacturing facility in Andover, Massachusetts
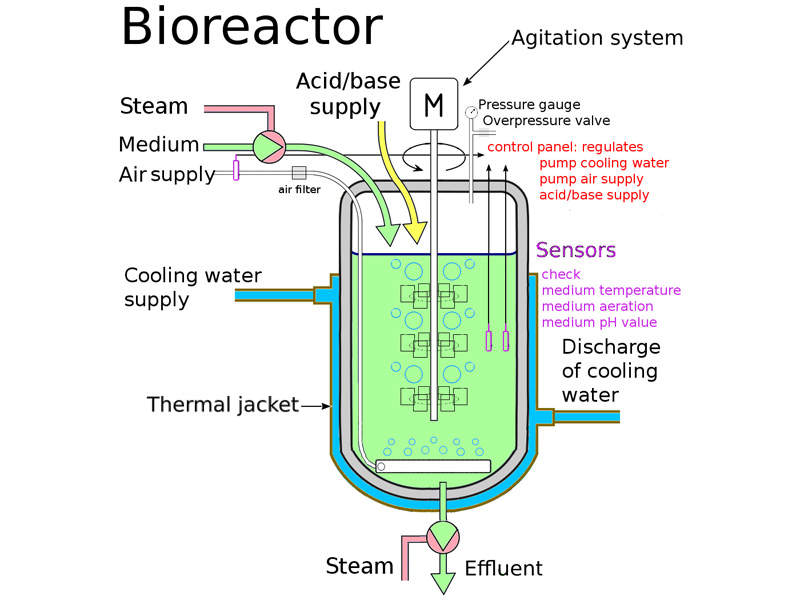
The facility will be a five-storey building with a 175,000ft² total floor space. It is designed to have five independent manufacturing suites to support Pfizer’s highly complex and diverse biologics research efforts.
The plant will be equipped with a flexible design to fully enable next-generation manufacturing strategies. It will use sophisticated single-use bioreactors and disposable process technologies.
Location of the Pfizer manufacturing facility in Andover
The manufacturing facility is being built as an expansion to Pfizer’s existing manufacturing facility in Andover, Massachusetts. The new facility will increase the campus’ capacity for producing complex biologics and vaccines.
The Andover facility is built on a 70-acre campus with seven buildings equipped with laboratories, clinical and commercial manufacturing suites and support areas. It also includes purification facilities and a clinical drug product manufacturing facility.
Andover is located in a strategically integrated site that serves an essential role in both Pfizer’s research and development (R&D) and global supply network. It has facilities for both R&D and flexible, multi-product manufacturing capabilities. It is a hub for R&D in Cambridge and has around 2,000 employees based in Massachusetts.
Massachusetts has attracted major investments from pharmaceutical companies, including Bristol-Myers Squibb‘s $280m plant in Devens and several other firms that have opened research centres in Cambridge.
Products manufactured at the Pfizer facility in Andover, Massachusetts
The facility will be used to produce vaccines and complex biologics, including BeneFIX®, Coagulation Factor IX (Recombinant) and bone morphogenetic protein (rhBMP). It will also produce polysaccharides, components of Prev(e)nar-13, and Pneumococcal 13-valent Conjugate Vaccine (Diptheria CRM197 Protein).
The Andover facility’s building B was approved as a multi-product facility that can allow clinical and commercial products to be produced simultaneously. The building was also designated as the launch site for Pfizer’s monoclonal antibody (mAb) drug substances.
Tax benefits of the facility
In May 2016, the Town of Andover approved a five-year tax-increment financing agreement for Pfizer at its annual town meeting. According to the agreement, Pfizer will pay tax at the current value for the first three years, then at 65% of the current value for the fourth and fifth years regardless of any increase in property value due to the new facility.
As a result of this, Pfizer will receive around $2.9m in tax breaks while generating roughly $3.9m in tax savings for Andover over ten years.
Marketing commentary on Pfizer
Pfizer is a global pharmaceutical company based in New York engaged in the discovery, development and manufacture of healthcare products. The company develops and manufactures medicines and vaccines for a wide range of medical disciplines, including immunology, oncology, cardiology, diabetology and neurology.
The company’s global portfolio includes medicines and vaccines, as well as many well-known consumer healthcare products.
A major part of Pfizer’s revenue comes from the vaccine business, which is mainly driven by strong uptake of its Prevnar 13 pneumococcal vaccine.