Aclaris Therapeutics’ Phase II ATI-502 for alopecia areata (AA) and Phase II for ATI-501 for AA, alopecia universalis (AU) and alopecia totalis (AT) may lack cosmetic significance due to their primary endpoints (key study measures).
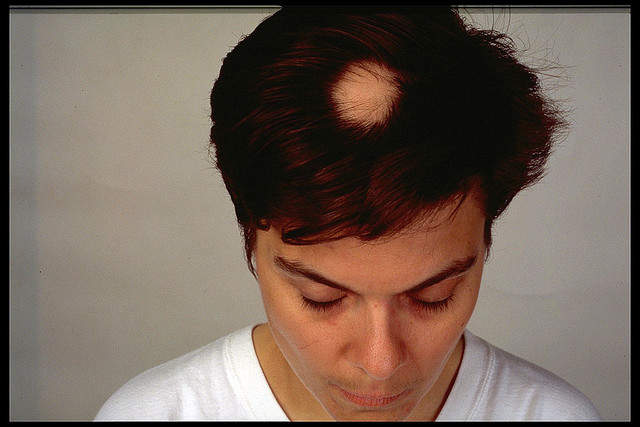
Alopecia areata, also known as spot baldness, is a condition in which hair is lost from some or all areas of the body. AA’s advanced form is AU, characterised by the complete loss of hair on the scalp and body. Another form of AA is AT, which is complete loss of hair on the scalp.
The primary endpoint of the topical ATI-502 study measures the amount of hair regrowth by the severity of the alopecia tool, as cited by ClinicalTrials.gov. Aclaris also notes the trial has pharmacokinetic (PK) and pharmacodynamic (PD) endpoints in a June press release. PK/PD are measurements of drug in the blood and body.
The trials’ primary endpoints are inadequate to gauge cosmetic significance, said three experts. The Severity of Alopecia Tool (SALT) is the primary endpoint of the oral ATI-501 study, according to ClinicalTrials.gov. The SALT score is a global severity score that captures the percentage hair loss.
SALT measures hair loss in different areas rather than the amount of hair regrowth, the latter of which is more relevant for cosmetic value, experts noted. Photographic images should be used to assess cosmetic significance, another expert said.
Experts also noted mixed views on whether the SALT primary endpoint in AT-501 will allow for clinical relevance considering the wider breath of skin area covered in the trial. Still, overall experts expect a positive reduction in hair loss for both topical ATI-502 and oral ATI-501 due to the clinical and real-world results from other JAK inhibitors, which is the class of both Aclaris therapies, and the preliminary positive Phase II biomarker (a measurable indicator of the severity or presence of some disease state) results for ATI-502.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataJAK inhibitors hinder the activity of the Janus Kinase family of enzymes which play a role in the cell signalling process that leads to the inflammatory and immune responses seen in conditions like alopecia.
Despite high efficacy expectations, experts also said the primary endpoint durations, at 12 weeks for topical ATI-502 (NCT03354637) and 24 weeks for oral ATI-501 (NCT03594227), are not long enough to assess long-term safety. An open-label extension (OLE) study of at least two years should be performed for both trials to assess safety, as opposed to the company’s six-month OLE plan for ATI-502, said experts. In particular, concerns about the potential development of squamous cell carcinoma remain due to the drug’s mechanism of action (MOA), said experts. A drug’s MOA usually includes the specific molecular targets to which the drug binds, such as an enzyme or receptor.
The full Phase II trial readout for ATI-502 is expected in the first half of 2019 and in the second half of 2019 for ATI-501, according to an Aclaris second quarter 2018 financial report.
Analysts predict positive results for both Phase II studies, with predicted combined peak sales of $1bn, with no peak year indicated. Aclaris’ market cap is $407m.
Aclaris declined to comment.
Cosmetic significance ultimately most desirable
Both the Phase II trials for ATI-502 and ATI-501 have elicited concerns on cosmetic significance. Despite the SALT’s clinical relevance in alopecia, the studies’ primary endpoints should have considered the amount of hair regrowth, said three experts. The alopecia tool score measures hair loss with a specific value before and after treatment, whereas determining the hair regrowth by counting hairs in a specific area pre- and post-therapy is closer to achieving cosmetic significance, three experts said.
Photographic imaging and patient satisfaction surveys should also have been co-primary endpoints in both studies to gauge cosmetic significance, said Dr Paul McAndrews, clinical professor, Department of Dermatology, University of Southern California School of Medicine, Pasadena.
Although the alopecia tool measurement determines clinical significance, cosmetic significance is more important for the patient as it is pertains to patient satisfaction about their treatment and any improvement in hair growth, said Dr Jason Reichenberg, clinical director of dermatology, University of Texas Southwestern, Austin and McAndrews. Even if there is 80% hair regrowth, this may not be cosmetically significant as it could mean only a few more strands of hair, said Reichenberg and McAndrews.
Experts declined or did not comment on the specific percentages and results expected for the SALT for both the ATI-502 and ATI-501 studies.
For ATI-501, which has a target population of AA, AU and AT, the SALT score, which measures the hair regrowth on the scalp, does not accurately measure the efficacy of the treatment on the back and the legs, said Dr David Perez-Meza, medical director, Perez-Meza Hair Institute, Malaga, Spain. Even a trial secondary endpoint, the Alopecia Density and Extent Score (ALODEX), measures hair regrowth in the scalp as well, said Perez-Meza. However, Yanling Liao, PhD, clinical assistant professor, Department of Pediatrics, New York Medical College, said it is a sufficient measure of hair regrowth even elsewhere in the body. Even though the primary endpoint is just measuring hair on the scalp, patients are more concerned about hair on their scalp than underarms or pubic regions, making SALT an adequate clinical measure, said Reichenberg and McAndrews.
Positive previous JAK, Phase II data bodes well
Earlier data on JAK inhibitors indicates they are clinically significant in treating AA, said Liao and Perez-Meza. They pointed to a pilot study with Incyte’s Jakafi (ruxolitinib), which showed that nine out of 12 patients (75%) treated with Jakafi had significant scalp hair regrowth and improvement of AA (Mackay-Wiggan et al. JCI Insight. 2016 Sep 22;1(15)).
Pfizer’s Xeljanz (tofacitinib), a JAK inhibitor approved for rheumatic arthritis and psoriatic arthritis, is being used off-label for AA treatment, said Reichenberg and McAndrews. Xeljanz has shown clinically significant results for patients, therefore ATI-502 and ATI-502 should have similar positive results, said McAndrews.
In addition, in interim Phase II data for ATI-502 in June, two of six patients had marked elevation of interferon gamma (IFN-γ) and cytotoxic T-cell (CTL) gene expression at baseline. After 28 days, the patient with the active treatment showed reduction in the two biomarkers, whilst the patient on vehicle did not demonstrate a positive change.
This data suggests that the MOA of ATI-502 works well in dampening the increase in the IFN- γ and CTL immune response, said Liao, Perez-Meza, Reichenberg and McAndrews. The results give confidence for further positive outcomes from both Phase II trials, as the MOA reduces the immune response, which is the cause of AA, they said.
However, although there was a reduction in the biomarkers on the patient treated with ATI-502, it is unclear whether the samples were taken from the blood or skin, said Dr Natasha Mesinkova, assistant professor, Department of Dermatology, UC Irvine School of Medicine, California. Reduction in the skin biomarkers would be needed to show ATI-502’s clinical significance, said Mesinkova.
Safety concerns need long-term assessment
Safety concerns linger about the prolonged effects of using JAK inhibitors, said Liao, Perez-Meza, Reichenberg, McAndrews and Dr Brett King, assistant professor of dermatology, Yale School of Medicine, New Haven, Connecticut. In particular, concerns on the risk of squamous cell carcinoma persist due its MOA, as JAK inhibitors can have severe systemic involvement in inhibiting immune signalling, resulting in cancer, said Liao, Reichenberg and McAndrews.
The most common adverse events associated with JAK inhibitors such as Jakafi are anaemia and thrombocytopenia, including the appearance of squamous cell carcinoma, said Liao, Reichenberg and McAndrews (Aboul-Fettouh et al. JAAD Case Rep. 2018 Jun; 4(5): 455–457).
Therefore, the safety assessment should be longer than 12 weeks and 24 weeks for the primary endpoint time frame for ATI-502 and ATI-501, respectively, said Liao, Reichenberg and McAndrews. The current OLE of six months should be up to two years to assess the long-term concerns, they said.
Topical ATI-502 will have fewer side effects compared to oral ATI-501, as it is less systemic, said Liao, Perez-Meza, Reichenberg and McAndrews. Interim Phase II ATI-502 data also suggested there is no systemic involvement, as indicated by plasma drug levels that were below the limits of quantification (1ng/ml) in all subjects at Day 28, said Liao, Perez-Meza, Reichenberg and McAndrews.
by Arafa Salam, PhD, in London
Arafa Salam, PhD, is a reporter for Pharmaceutical Technology parent company GlobalData’s investigative journalism team. A version of this article originally appeared on the Insights module of GlobalData’s Pharmaceutical Intelligence Center. To access more articles like this, visit GlobalData.