In the last year, well-known contract manufacturing organisations (CMOs) such as Agilent Technologies (Santa Clara, California) and Abzena (Cambridge, UK) have expanded their continuous manufacturing capabilities. US Congress is also making substantial investments to improve the continuous manufacturing of pharmaceuticals. As more drugs are approved with continuous manufacturing, this production method is becoming increasingly mainstream.
On 27 May, Agilent announced a collaboration with APC (Dublin, Ireland) on automated process analysis via liquid chromatography. Allowing for real-time process monitoring during drug production can help with continuous manufacturing. Abzena is expected to open a continuous manufacturing facility for biologics in Sanford, North Carolina, in Q4 2022. National Resilience (San Diego, California) announced on 6 June that it had raised $625m in series D financing, which will be spent in part on continuous manufacturing for biologics.
On 3 September last year, the US Food and Drug Administration (FDA) released draft guidance on continuous manufacturing of active pharmaceutical ingredients (APIs) and finished dose drugs, including conversion of batch manufacturing to continuous manufacturing for existing products, named ‘Q13 Continuous Manufacturing of Drug Substances and Drug Products’. The US House of Representatives separately passed a bill on continuous manufacturing on 19 October last year. The ‘National Centres of Excellence in Continuous Pharmaceutical Manufacturing Act of 2021’ (H.R. 4369) directs the FDA to spend $100m on creating continuous manufacturing centres within universities over the next four years. The bill received bipartisan House support and has passed to the Senate Committee on Health, Education, Labour and Pensions.
Larger CMOs have been increasingly adopting the use of continuous manufacturing for biologics production, following the first FDA approvals of biologics continuously manufactured dose products last year. The contract manufacturing industry has been discussing incorporating these techniques for the last five years, but the uptake of continuous manufacturing has been more gradual than predicted.
Continuous manufacturing is a process in which the input materials are continuously fed into and transformed, and the output materials are continuously removed from the system. One of the oldest continuous operations is simulated moving bed chromatography, the basis of which can be tracked to early patents from the 1950s. Continuous manufacturing of small molecule drugs came first, before that of biologics; the first related approval was in 2015 for Vertex Pharmaceuticals’ (Boston, Massachusetts) Orkambi (lumacaftor/ivacaftor) to treat cystic fibrosis.
It is now time for the next frontier in terms of increasingly complex molecule types. Janssen’s (Titusville, New Jersey) Prezista (darunavir), another small molecule drug that is used to treat human immunodeficiency virus (HIV), was the first drug that the FDA allowed to be switched from batch manufacturing to continuous manufacturing. There are currently ten approved continuous manufacturing applications in the US.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData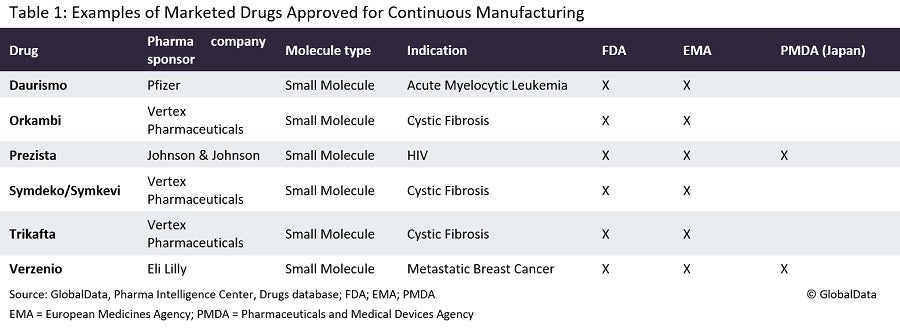
In a CPhI North America session called ‘Continuous Manufacturing. What’s Driving Adoption?’, Bikash Chatterjee, CEO at Pharmatech Associates, a USP company, discussed the advantages of continuous manufacturing. He stated: “On average, folks that did continuous manufacturing had eight months faster approval time and 12 months faster from submission to market; to me, that is very compelling.”
Chatterjee continued: “In a marketplace where business performance is paramount given economic pressures, pricing and reimbursement, that’s not going away. Continuous manufacturing represents a tremendous solution for a lot of drug product manufacturers and drug sponsors.”
There are still considerable issues that will prohibit the widescale uptake of continuous manufacturing, such as a certain level of unpredictability and a lack of reproducible results, which is in part contributed to by a current lack of standardisation of equipment and software.
In the same CPhI North America session, William A Hein II, Senior Director of Technical Operations, Small Molecule Platform at Janssen, commented on standardisation: “There’s a lot of industry collaboration but I do not think there is a standard set of solutions yet available to us as an industry. There are options and solutions out there and there is a lot of customisation that’s going on. We are in an age where we are talking about software; in an ideal world, it would be great if there was a platform like iPhone or Android and you can bring your equipment in and connect it, but we are not there yet.”
There are many advantages to continuous manufacturing; for instance, batch manufacturing, which constitutes the majority of manufacturing, takes more time to perform, and there are also reduced costs, potential improvement of quality and a reduction in the likelihood of human error. Despite these potential benefits, the industry has been slow to adopt these techniques, but it seems inevitable, as greater understanding develops and improved equipment becomes available for continuous manufacturing, that this will become the new standard in future.




