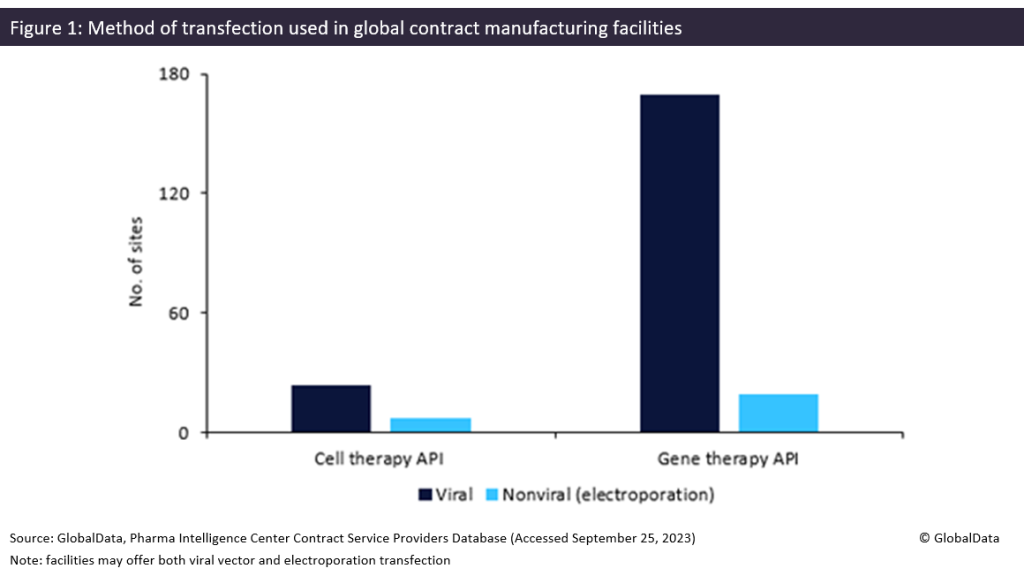
Viral vectors are commonly used in the manufacture of cell and gene therapies, but come with high manufacturing costs and capacity constraints. Although more rarely used for transfection, non-viral vectors could alleviate these problems, experts have said.
Cell and gene therapies have drawn huge interest from manufacturers, investors and developers alike, but their developers still face manufacturing bottlenecks due to a lack of manufacturing facilities with the relevant equipment, know-how or raw materials.
There are three methods of transfection: biologic (involving viral vectors), chemical and physical (such as electroporation).
Viral vectors are very efficient at transducing genetic cargo into host cells; this method of transfection has been widely used and well-proven over the past few decades. However, viral vectors can present complex manufacturing challenges, contributing to high production costs and difficulty when manufacturing on a commercial scale. Viral vectors can also have problems with relatively low packaging capacity (for adeno-associated viruses it can be less than 5 kilobases), immunogenicity and oncogenicity.
Chemical transfection can be liposomal-based or non-liposomal-based. Electroporation is currently the most widely used physical method of transfection. It uses an electrical pulse to create reversible pores in cell membranes through which nucleic acids can enter. It can work with a diverse range of cell types, including difficult-to-transfect cells such as B-cell lines, primary cells and stem cells, can operate in living organisms and is highly efficient. However, due to the high voltage pulses used, cell death or incomplete membrane repair can occur, as well as nonspecific transport of molecules.
To overcome the limitations associated with viral vectors, the biopharma industry is turning to nonviral delivery systems, both physical and chemical. Non-viral vectors are more easily manufactured. They could therefore make cell and gene therapies cheaper and improve their uptake with healthcare providers and patients. Speaking at the Phacilitate conference in Portugal in September 2023, at a session called “Progressing MSAT: Exploring Development, Standards and Standardisation to Ensure Manufacturing Technologies for Commercial Production”, Pedro Silva Couto, a postdoctoral fellow at University College London, elaborated on the problems of producing CAR T cell therapies with viral vectors.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataPedro stated: “One of the main disadvantages of viral vectors is related to their difficulty in manufacturing dose at scale. In addition to that, the lengthy process and the retrovirus are responsible for a significant fraction of the costs associated with CAR T, and that’s why we thought at UCL that the non-viral approach would be a suitable strategy to decrease the cost of manufacturing associated CAR T products. Non-viral really has the potential to tap into not only autologous manufacturing but also allogeneic manufacturing.”
Currently, the manufacture of CAR T cells mostly relies on γ-retroviral or lentiviral vectors. Academia and big pharma are exploring non-viral techniques such as membrane permeabilisation and carrier-based techniques as potential replacements.
Contract manufacturers could find an industry shift towards nonviral transfection problematic. As shown in Figure 1 below, most transfection facilities offer viral methods rather than electroporation. According to the Contract Service Provider database, Patheon (Waltham, MA, US) and Catalent (Somerset, NJ, US) have the most gene therapy sites offering transfection, with six each.