Combinations involving checkpoint therapies are starting to be used as the standard of care in certain metastatic cancer types, such as Merck’s Keytruda (pembrolizumab) with chemotherapy in advanced squamous non-small cell lung cancer.
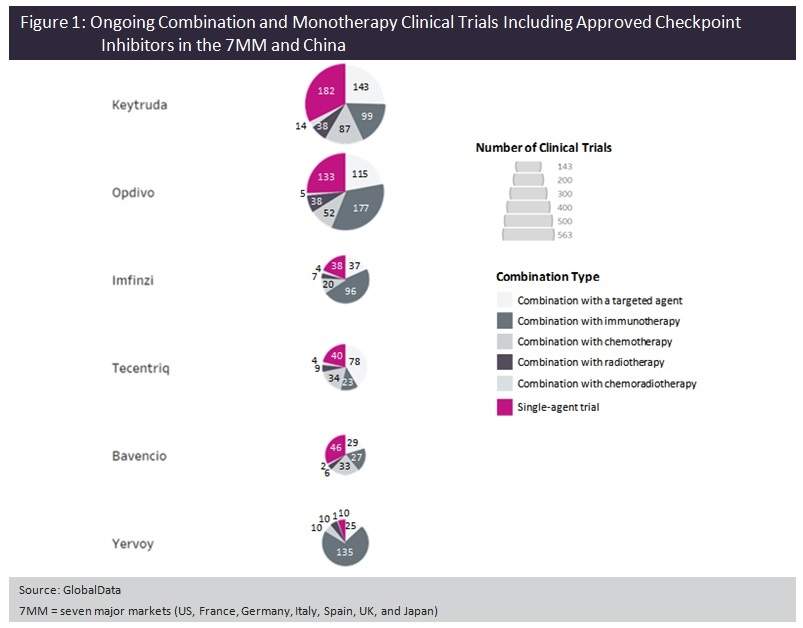
Analysis by GlobalData of the six approved checkpoint inhibitors—Keytruda, Bristol-Myers Squibb’s Opdivo (nivolumab) and Yervoy (ipilimumab), AstraZeneca’s Imfinzi (durvalumab), Roche’s Tecentriq (atezolizumab), and Merck KGaA/Pfizer’s Bavencio (avelumab)—has shown that in fact the majority of ongoing Phase I to Phase III clinical trials involve a combination regimen.
This reflects the need in many cancer types to improve upon the efficacy achieved with checkpoint agents as monotherapies, and to better serve the substantial number of patients who do not respond to checkpoint agents alone. Although the increased cost of adding other treatment modalities to already expensive checkpoint inhibitors could present a significant barrier to market access, the trend for combination trials shows no sign of slowing at present.
All six of the marketed checkpoint modulators are being studied in trials in combination with several other types of therapy: immuno-oncology (IO) agents, non-IO targeted agents (defined by GlobalData as small molecules or biologics with a non-immune-related target), chemotherapy, radiotherapy, or chemoradiotherapy.
The most common types of combination partners for all six approved checkpoint inhibitors are either non-IO targeted agents or other IO agents, with Opdivo, Imfinzi and Yervoy having notably large numbers of ongoing studies with other IO agents.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataNo single type of combination including checkpoint agents has been proven so far to be a superior option across cancer indications, suggesting broad opportunities for developers to test their checkpoint assets in multiple indications and combinations. Indeed, among the 115 checkpoint inhibitors currently being investigated in the clinic, differentiation from the six approved agents will be key to their success, and combination strategies may be the best way to achieve this.