Enhanced Efficiency with Platina QMS
Kemwell offers contract manufacturing of pharmaceutical products for global distribution.

Formpipe specialises in developing enterprise content management (ECM) software, enterprise quality management solutions (eQMS), and electronic information organisation programmes for a global client base in the pharmaceutical industry.
You have successfully submitted your enquiry. Someone from our company will respond ASAP

Formpipe provides enterprise quality management solutions for a global client base in the pharmaceutical and life science industries.
Platina QMS by Formpipe is a fully integrated enterprise quality management software (EQMS) solution that allows users to have total control.
One of the most regulated industries is life sciences, covering every stage from development and trials, through to manufacturing and delivery.
In addition to the growing regulation requirements, the complexities encountered in this industry need to be continuously adapted to. While some companies find themselves struggling with mountains of paper-based systems, our clients benefit significantly from document organisation software. Constant changes can be adapted to with ease through a comprehensive, easy-to-use information management system.
From using the system when the information is collected, lengthily procedures such as reviews and reports are considerably easier to manage.
Other benefits of using Platina QMS include:
Whether you are looking for a system to manage regulatory documents, SOPs, corrective and preventative actions (CAPA), change control, or training, Platina QMS offers a comprehensive, user-friendly and ready-to-use software solution that facilitates easy creation, management and tracking of all quality-related documents, records, events and processes
The flexible and web-based system comes with pre-defined and ready-to-use modules, such as:
Platina QMS has been developed in accordance with Formpipes’ stringent quality management systems, ensuring the meeting of US and EU GMP standards for manufacturing in the pharmaceutical and life sciences industries.
Based on the Platina system, Platina QMS is an advanced vertical application. On a daily basis, Platina is used by thousands of industry professionals, encompassing those working in life sciences, healthcare and government agencies.
Platform users enjoy full control and traceability, combined with a holistic and cost-effective business solution.
Platina QMS is highly efficient, ensuring safety and regulatory compliance. It combines management of documents, cases and training with instant reports and has a considerable process engine for automated workflows.
Regulatory compliance can be assured throughout an entire business, from conception and development, to manufacturing and distribution.
Platina QMS provides full support for meeting regulatory compliance with FDA Title 21 CFR Part 11 and EU Annex 11 requirements. This involves data and integrity, traceability / audit, and electronic signatures. The flexible software solution enables the automation of routine operations and improves efficiency on every level.
Our solutions reduce costs, minimise risks, decrease lead times and improve quality control.
Formpipe develops products for structuring information and supplies these to major companies, authorities and organisations.
Our software helps organisations capture, manage and distribute data, and place it in context. Reduced costs, minimised risk exposure and structured information are all benefits that can be gained by using our products.
The company was founded in 2004. Formpipe Software’s share is listed on Nasdaq OMX Nordic, Small Cap.

Kemwell offers contract manufacturing of pharmaceutical products for global distribution.

The constantly changing market within the life science industry calls for companies to constantly improve their performance and quality outcomes in order to stay competitive.

Platina QMS is a fully integrated electronic quality management system solution (EQMS) that maximises both control and efficiency.

With Platina QMS by Formpipe, business documents and processes are streamlined and structured to strengthen quality management, maximise control and help improve performance and profitability.

US-based pharmaceutical company Emulate inaugurated a new headquarters and laboratory in Seaport District in Boston, Massachusetts, in February 2016.

Formpipe acquires the UK company GXP Limited (GXPi), an established provider of compliance solutions for the life science industry.

FormPipe Software has received a SEK 2.5m order from Recipharm for its electronic quality management system (EQMS), FormPipe Life Science. The software is designed to streamline quality and compliance processes.

Contract pharmaceutical company Kemwell has placed a SEK 2m additional module licence order for FormPipe’s ECM product, Platina. The order will be fulfilled by FormPipe’s parner, Sigma.

To ensure quality and efficiency when developing and operating processes, it is vital to have an optimised corrective and preventive actions (CAPA) management system.

One of the most important elements in a life science organisation's quality management system is Change Control. Substantial non-compliance risks could arise should change control procedures be insufficient.
It is important to manage any deviations in expected standards in the development, manufacturing and distribution processes of pharmaceutical products.

Standard operating procedure (SOPs) and other important documents require management throughout their lifecycle, from creation and review to formal usage and final withdrawal.
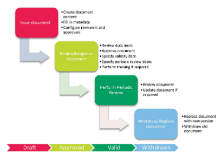
In FDA-regulated industries, such as pharmaceutical and life sciences, standard operating procedures (SOPs) must be managed with strict change control processes to assure compliance with current GxP quality guidelines and regulations.
Regardless of company size, it is vital that staff are trained in programmes for quality control, risk and compliance.