Hard Capsule Vendor Change - Annual Report
This MAPP establishes the policy in the Office of Pharmaceutical Quality (OPQ) for a postapproval change in the supplier of a hard gelatin capsule shell.

EMBO CAPS® is a range of highly customisable and secure encapsulation technologies that allow drug developers to achieve wide product diversification.
You have successfully submitted your enquiry. Someone from our company will respond ASAP
EMBO CAPS® is a range of highly customisable and secure encapsulation technologies that allow drug developers to achieve wide product diversification.
The solutions provide a variety of different drug delivery solutions, including plant fibre-cellulose capsules for patients with diet restrictions, capsules designed to minimise brittleness for moisture-sensitive or hygroscopic dosages, sodium lauryl sulfate (SLS) free capsules, and low-powder retention capsules for dry powder inhalation.
The EMBO CAPS® range incorporates a patented design that maximises capsule performance and function. They are developed with a pre-lock size and configuration that minimises premature separation during shipping and storage, while maximising filling machine separation performance.
The capsule is also designed to prevent it from moving to the lock position from pre-lock during shipping.
The design also includes a computer-engineered dome radius that directs the closing force along the side wall of the shell to reduce denting or dimpling during capsule filling. The amount of air allowed to escape when closing is also optimised to provide a uniform closed capsule length, and the mechanical hoop strength of the cap is increased.
In addition, the capsules have a double-lock and a zone of constant diameter to ensure the cap is engaged correctly.
EMBO CAPS® capsules are manufactured to optimise stability and do not need to be refrigerated in transport.
The range utilises high-grade raw materials, including gelatine that is sourced from Suheung subsidiary Geltech. In addition, stringent specification compliance and computerised raw material cross-blending ensure only powders that meet the exact requirements can reach the capsulation machines.
EMBO CAPS® carry a five-year expiration date and meet regulatory requirements for the pharmaceutical market.
Since its foundation in 1973, Suheung has focused on offering high-quality, empty capsules.
The company has its own equipment manufacturing capabilities and production sites, and the uniformity of its capsules is ensured by state-of-the-art, automated robotic and vision inspection systems.
Suheung also provides contract manufacturing services for the pharmaceutical and nutraceutical industries, presenting a wide range of dosage types and packaging options.

This MAPP establishes the policy in the Office of Pharmaceutical Quality (OPQ) for a postapproval change in the supplier of a hard gelatin capsule shell.

The purpose of this MAPP is to establish the policy in the Office of Pharmaceutical Quality (OPQ) for a postapproval change in the supplier of a hard gelatin capsule shell.

The purpose of this white paper is to compare the equivalence of empty pharmaceutical hard capsules with various traditional raw materials such as gelatin and non-traditional vegetable hydrocolloids.
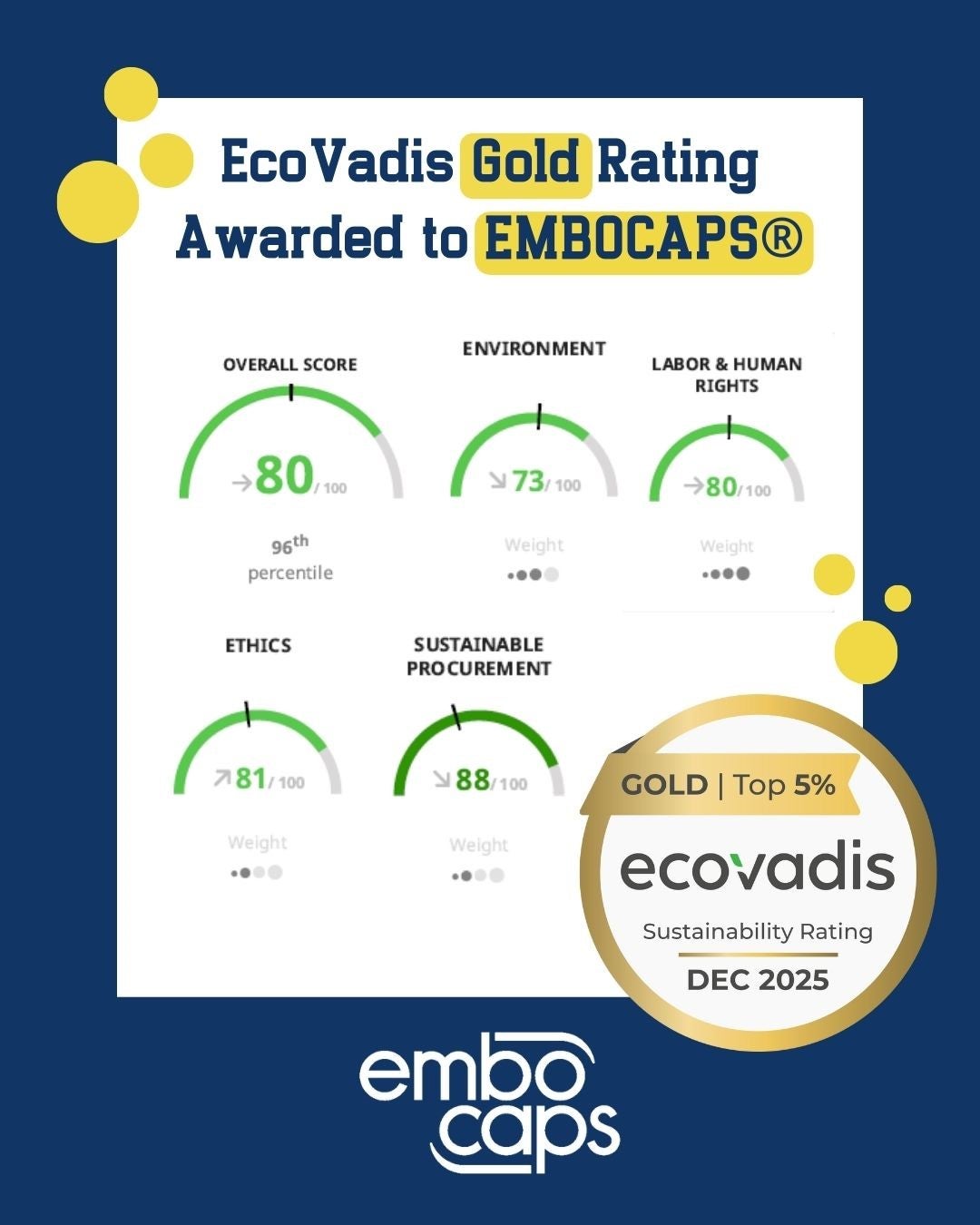
We’re proud to share that EMBOCAPS® has once again been awarded the EcoVadis Gold rating, following last year’s recognition.

EMBOCAPS® FLEX #00F and #0F offer a smart alternative—one capsule that allows you to produce both elongated and standard-size products with a single solution.

EMBOCAPS® is heading to INTERPHEX WEEK TOKYO 2025—just around the corner.

The EMBOCAPS® AP60 is perfect solution for special ingredients targeted to reach the colon or pH sensitive fill materials. This capsule survives the harsh conditions encountered in the upper part of the gastrointestinal (GI) tract for more than 60 minutes.

Suheung Capsule has announced it will be attending CPhI Worldwide.

Suheung America Corporation has announced the release of the new EMBO CAPS® VG-ALPHA drug delivery system, an empty vegetarian capsule targeted for use in the pharmaceutical market.

EMBO CAPS® manufactures pharmaceutical capsules using a proprietary blend of Type B pharmaceutical-grade bovine and Type A pharmaceutical-grade porcine gelatines.
Manufactured using pharmaceutical-grade fish gelatine, EMBO CAPS® Fish gelatin capsule provides an excellent oxygen barrier and a crystal clear capsule shell.

EMBO CAPS® VG Hypromellose is a non-animal alternative capsule for pharmaceuticals.

EMBO CAPS® VG Alpha is a vegetarian capsule that is free from genetically modified organisms (GMO) and is manufactured using hypromellose.

EMBO CAPS® AP is a non-animal delayed-release capsule.