Beate Beime Pharma Consulting
Readability Tests for Pharmaceutical and Medical Device Applications
Beate Beime Pharma Consulting supports pharmaceutical and medical device developers throughout the regulatory approval process to ensure that labelling and medical documents meet stringent industry regulations.
Subscribed
You have successfully submitted your enquiry. Someone from our company will respond ASAP
About Us

Beate Beime Pharma Consulting supports pharmaceutical and medical device developers throughout the regulatory approval process to ensure that labelling and medical documents meet stringent industry regulations.
The company develops a systematic approval strategy comprising readability tests and consulting services to prove the efficacy and tolerability of products with an optimal use of resources.
Consulting service for medical device and drug regulatory applications
Beate Beime Pharma Consulting develops customised strategies for drug and medical device manufacturers to ensure that development processes meet regulatory requirements in Europe.
The company has a comprehensive understanding of the regulatory application process, including requirements for aspects such as preclinical development, clinical trial management and document preparation.
Beate Beime Pharma Consulting’s service includes:
- Advice on clinical trials in the approval process
- Development of approval strategies, analysing regulatory options and barriers
- Authority talks
- Creation of documentation such as preclinical and clinical approval reports, investigational medicinal product dossiers (IMPD), test plans, case report forms (CRF) and final reports
- Paediatric medicine support, including the creation of paediatric investigation plans (PIP) and preparation for paediatrics-use marketing authorisation (PUMA) applications
- Ensuring labelling meets regulatory requirements
Project management and planning for clinical studies
Beate Beime Pharma Consulting collaborates closely with certified clinical research organisations (CRO), biometrics and physicians to formulate, coordinate and monitor drug tolerability and efficacy clinical trials.
The company’s experienced project management team supports clients throughout the clinical development process, from strategy and planning to the preparation of results for regulatory applications.
The firm also prepares PIPs for medicines being developed for children.
Readability tests for pharmaceutical packaging leaflets and labelling documents
Beate Beime Pharma Consulting conducts readability tests on a wide range of documentation in preparation for regulatory applications for medical devices and pharmaceuticals.
This validated and standardised test method ensures documents related to clinical trials, drug and device development, and packaging leaflets are easy-to-read and understand. Tests are performed quickly and efficiently to meet application deadlines.
Beate Beime Pharma Consulting’s readability tests include:
- Single interviews with 20 subjects from the drug or medical device’s target group
- Evaluations after each interview allowing for quick correction of difficult-to-read sections
- Documents are prepared for submission to the CTD Module 1.3.4
Tests can be conducted in English, German, Romanian and Croatian. Text comparisons, bridging studies and focus tests are also available.
User testing for drugs and medical devices
Beate Beime Pharma Consulting provides a wide range of user tests for drugs and medical devices, including divisibility tests, acceptance tests for new dosage forms, taste tests for paediatric medicines, and application tests for medical devices.
The company also conducts readability, comprehensibility and applicability studies for educational materials and clinical study documents such as patient information, diaries and questionnaires.
Beate Beime Pharma Consulting tests documents such as:
- Training materials for professionals and patients
- Information leaflets for patients such as brochures, guides and checklists
- Instructions for use (IFU) for medical devices
- Patient support and compliance programme (PSP) apps, software and websites
- Clinical trial descriptions and patient information sheets (PIS)
- Informed consent forms (ICF)
- Patient diaries
- Surveys to collect patient assessments in clinical trials (quality of life survey, perceived disease symptoms, patient-reported outcomes)
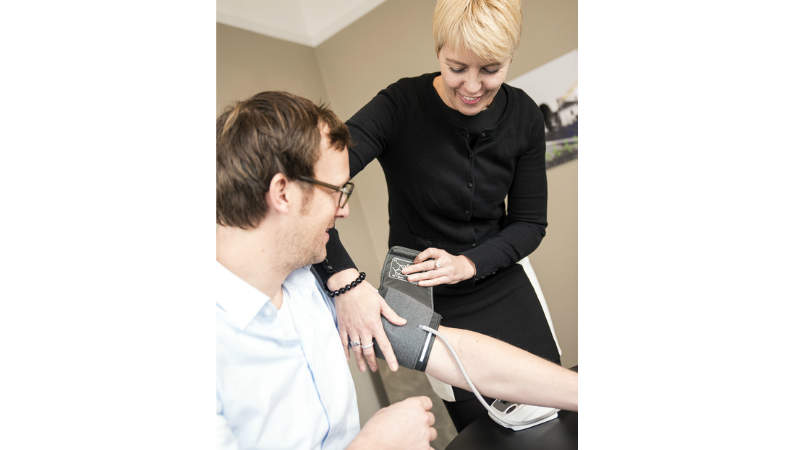
Accuracy studies for medical device validation
Beate Beime Pharma Consulting provides clinical studies at IPPMed for the validation of sphygmomanometers, meeting protocols such as European Society of Hypertension (2010), German High-Pressure League (2010), British Society of Hypertension (1993) and ISO: 81060-2.
Contact Details
Website
Email Address
Address
26135 Oldenburg,
Germany