Matesy offers clinical and preclinical research services in the field of drug absorption studies. The magnetic capsule MAARS, in combination with the magnetic monitoring system 3D-MAGMA, allows drugs to be delivered precisely into the gastrointestinal tract in humans and animals for HDA as well as very early proof-of-concept studies at very low cost levels. Within our Pharmagnetic network or directly with the principal we are able to serve drug development solutions at different stages.
Tracking magnetic marked capsules in the gastrointestinal tract
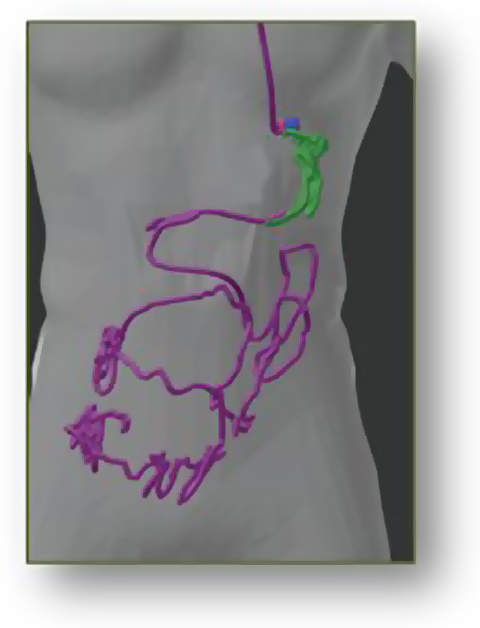
3D-MAGMA is a device for real-time tracking of magnetic marked capsules inside the human gastrointestinal tract. The localisation accuracy gives a 3D position with a maximum error of less than 3mm and allows the 3D path of the capsule to be calculated. Thus, the user always knows the capsule’s exact position.
Tracking magnetic marked capsules without radioactive tracers
3D-MAGMA is a CE-certified medical device. Its main features are:
- Accurate real-time position acquisition
- Works without ionising radiation
- Correlation of 3D position and position within the organ
- Measuring of travelled capsule path and motility patterns
Magnetic capsule for in vivo pharmacokinetics and drug metabolism
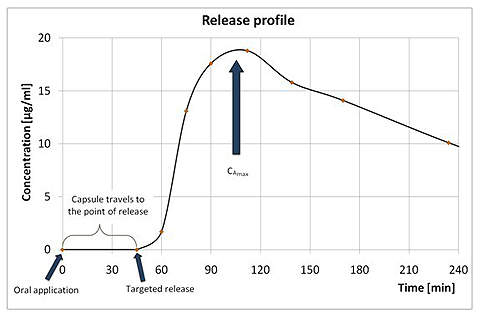
MAARS is a remote-controlled magnetic capsule that offers controlled release of any dosage form of active agents into the GI-tract far before galenics must act. The scalable MAARS capsule transports up to 2.0ml of the active agent safely to the targeted region and releases all of the content, triggered by a short magnetic impulse. Behaviour of the active agent can be studied with standard methods.
The main features of drug delivery with the MAARS remote-controlled magnetic capsule are:
- Completely passive magnetic capsule inside the organism
- Large payloads of up to 2.0ml possible
- Release triggered by short magnetic impulse
- Complete release
- Small capsule elements after release for an easy withdrawal
In vitro metabolism with a magnetic capsule and magnetic monitoring system
The MAARS and 3D-MAGMA technology offers two key capabilities in the field of in vitro metabolism: identifying drug interaction risks, and elucidating idiosyncratic risks and transporter issues.
ADME programmes include:
- Kinetics for drugs and standard agents
- Absorption windows
- Drug release characteristics
- Intestinal drug targeting
- Metabolite profiling and identification
Benefits of a remote-controlled magnetic capsule and magnetic monitoring system
Using the MAARS remote-controlled magnetic capsule and the 3D-MAGMA magnetic monitoring system enables the release of arbitrary drug formulations at any destination inside the complete gastrointestinal tract of humans and animals.
MAARS and 3D-MAGMA have preclinical and clinical applications due to scalable capsules, and both solid and liquid dosage forms are possible. No radioactive marker is necessary, and the MAARS and 3D-MAGMA technology offers low stress for volunteers and clinical personnel. It also offers fast results with moderate effort.
Services for human drug absorption studies
As part of its portfolio of services Matesy can execute preclinical and clinical resorption trials. The Pharmagnetic network covers very early proof-of-concept studies, even before development of a preliminary formulation, as well as the import and release of test medication according to EU regulations. Within the network we also handle in vivo and ex vivo pharmacokinetic and drug metabolism studies.
Please contact us to discuss your specific needs with one of our specialists.