Located in Waukegan, Illinois, Pfanstiehl is a leading current good manufacturing practice (cGMP) manufacturer specialising in parenteral-grade excipients and highly potent active pharmaceutical ingredients (API). With over 100 years of experience, Pfanstiehl plays a vital role in supplying High-Purity, Low-Endotoxin, Low Metals (HPLE-LM™) carbohydrates and amino acids to the pharmaceutical industry
High-purity, low-endotoxin carbohydrates
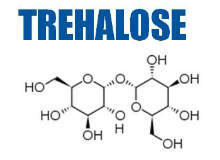
- Trehalose
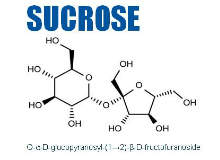
- Sucrose
- Galactose
- Mannitol
- Maltose
- Mannose
High-purity, low-endotoxin amino acids
- L-Arginine
- L-Arginine HCL
- L-Histitidine
- L-Histidine HCL
- L-Methionine
- L-Glutamine
Many of these carbohydrates are essential for the stabilisation of proteins, monoclonal antibodies (mAbs), and vaccines, making them a crucial component in injectable medications. Pfanstiehl collaborates with top pharmaceutical and biopharmaceutical companies around the globe, producing proprietary and commercial compounds in quantities ranging from grams to tonnes.
Customised manufacturing solutions
Whether you are developing a novel formulation as an innovator or replicating an existing one as a biosimilar firm, Pfanstiehl provides a reliable, high-purity solution. They specialise in custom synthesis of new chemical entities (NCEs), speciality carbohydrates, and injectable-grade cGMP pharmaceutical ingredients in commercial quantities. Pfanstiehl’s capabilities include process scale-up, development, and validation according to strict cGMP guidelines.
The company’s facilities are equipped to handle the production of HPLE compounds, including highly potent drugs, under cGMP conditions. Pfanstiehl also offers invaluable support in managing the regulatory process, establishing and maintaining both US and European Drug Master Files (DMFs) and European Certificates of Suitability, ensuring that your product is market-ready.
cGMP expertise and compliance
Pfanstiehl’s FDA-registered and inspected facilities are designed to meet the highest regulatory standards. With more than 15 active DMFs across the US, Canada and Europe, their cGMP manufacturing processes ensure the highest quality standards. The company’s fully qualified equipment and utilities in their cGMP manufacturing areas guarantee compliance, backed by robust validation and qualification systems.
As a trusted manufacturer of low-endotoxin carbohydrates, injectable-grade excipients, APIs, and pharmaceutical intermediates, Pfanstiehl is committed to helping its partners seamlessly bridge the critical gap between product development and regulatory approval, offering end-to-end solutions that ensure your compounds reach the market with confidence.
Quality, purity and safety in everything we do
Pfanstiehl’s extensive experience, combined with its state-of-the-art cGMP facilities and regulatory expertise, makes it a preferred partner for pharmaceutical firms worldwide. From custom synthesis to regulatory support, Pfanstiehl is dedicated to delivering high-purity, injectable-quality solutions that meet the most stringent industry standards.