ZETA's Perspective on Enhancing Buffer Management Concepts
There is a noticeable trend towards capacity expansion and process intensification for cell culture and fermentation processes.

ZETA specialises in designing, constructing, automating, and qualifying customer-specific aseptic production systems, while also acting as an EPCM contractor for major pharmaceutical projects.
You have successfully submitted your enquiry. Someone from our company will respond ASAP

ZETA is a globally operating end-to-end solution provider for the biotech and pharmaceutical industries.
The path of newly developed active substances in the lab into the preclinical phase and in early clinical GxP production, followed by scaling of the manufacturing process to industrial scale, through to market introduction in secure routine operation at the production facility, is a complex and challenging process for the pharma and biotech industry. Along with this entire active substance development and manufacturing process, ZETA offers comprehensive services and offers solutions for the construction, scaling and upgrade of production equipment.
ZETA provides support after process equipment commissioning through targeted ramp-up, as well as service and maintenance along the entire product lifecycle. True to the principle of continuous improvement, ZETA guides customers during process and system optimisation as well as operator training. ZETA’s aim is to achieve a 100% batch success rate.
As an EPCMV expert, ZETA manages the complexity of large-scale projects — from process plant design and construction to cleanroom planning and technical building services — while actively reducing interface risks. ZETA’s design-build approach accelerates project execution by up to 50%, enabling earlier value creation in customers’ CAPEX projects. In doing so, ZETA contributes to the faster market launch of life-saving active ingredients.
ZETA has established itself as an innovation driver in the pharmaceutical and biotech sectors – particularly through the implementation of a fully integrated digital tool chain, encompassing process simulation, engineering, qualification, and operator management. Process simulation plays a key role by enabling early, data-driven decisions, minimising risks, and significantly increasing efficiency in both planning and operations.
Together for a sustainable future in the pharma and biotech industry
ZETA is also actively taking steps towards a more sustainable future for the entire industry. Holistic decarbonisation strategies that reduce targeted emissions during the biopharmaceutical manufacturing process are integrated with sustainable energy sources for the power supply of the entire infrastructure. Investment scenarios in renewable energy are evaluated using profitability calculations.
ZETA is a globally operating end-to-end solution provider for the biotech and pharmaceutical industries:

There is a noticeable trend towards capacity expansion and process intensification for cell culture and fermentation processes.
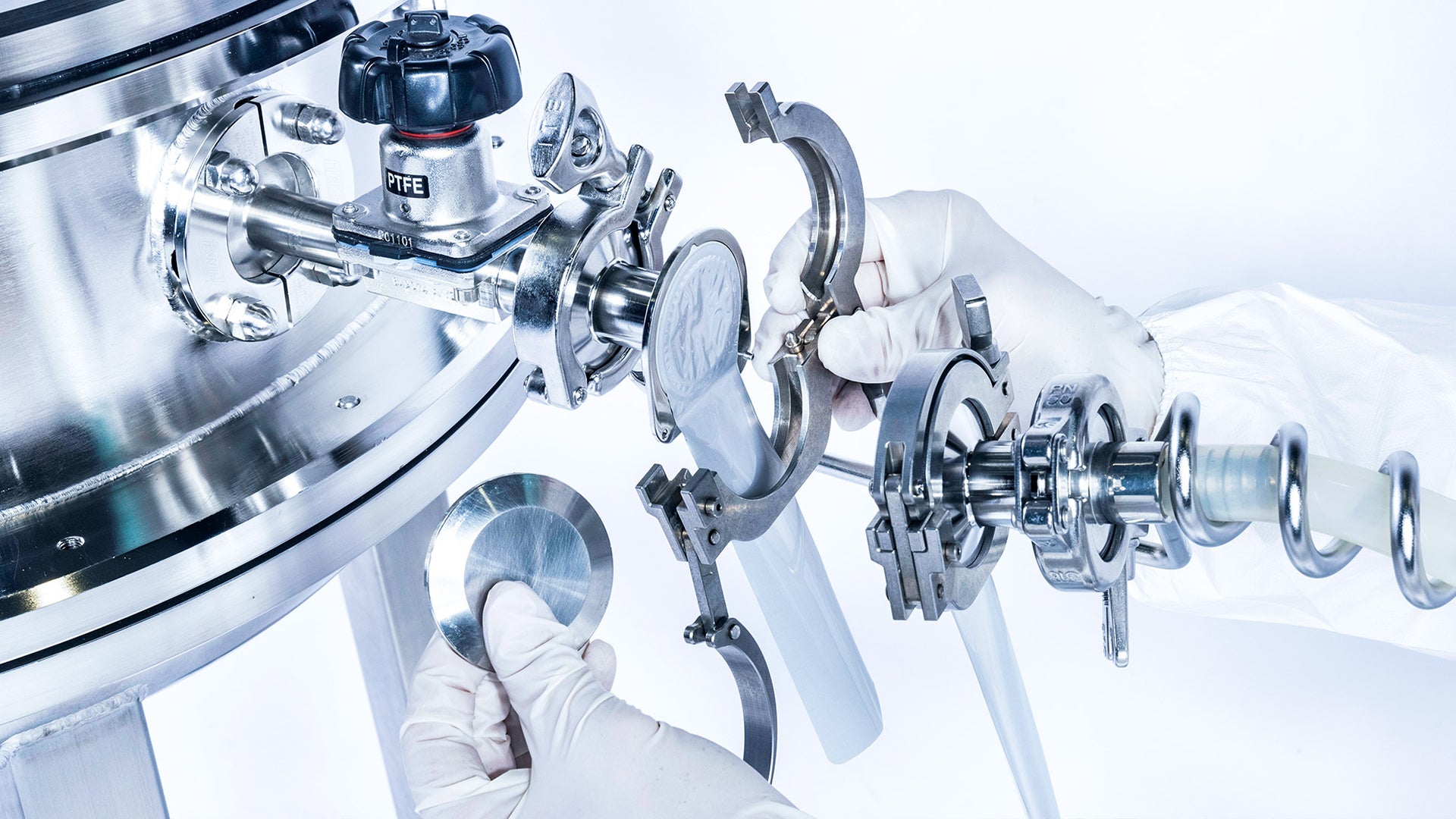
With EcoConnect, ZETA offers a reliable and environmentally friendly connection solution for the transfer of liquids. Georg Pöschl gives us an insight into the innovative technology and its development.

Since 2011, ZETA has been delivering customised single-use (SU) systems such as bioreactors, chromatography and filtration systems, mixers, and many others to customers around the world.

ZETA provides the best-in-class services and customised solutions to the specific problems of its customers.

The design of agitators for mammalian cell cultures must provide sufficient power input for effective mixing, heat transfer, oxygenation and CO2 stripping while minimising shear forces to prevent cell damage. ZETA has developed an optimal agitator configuration tailored to low shear systems.

The pharmaceutical industry is under increasing pressure to expand production capacities quickly and efficiently – often while operations are ongoing. Brownfield projects, where existing facilities are extended or modified, are particularly demanding. ZETA addresses these challenges with an integrated engineering approach in which process simulation plays a central role.

Pharmaceutical and biotech facilities are complex environments that demand precise coordination of product diversity, material flows, and personnel resources. In this interview, Peer Sander, Head of Technical Affairs at INOSIM, explains how the predictive decision support system INOSIM Foresight is applied in practice and what tangible improvements it delivers.

The demands placed on modern production facilities in the pharmaceutical and biotech industries are as diverse as the therapies they enable.

ZETA – end-to-end solution provider for the pharmaceutical industry – is continuing its global expansion and has opened a new subsidiary, ZETA Solutions LLC in Almaty, Kazakhstan, in August 2024.

ZETA, a leading provider of end-to-end solutions for the biopharma and pharma industry, has announced the acquisition of a majority stake in INOSIM Software GmbH, a German company that specialises in software solutions for the optimisation of production processes.

ZETA, a leading international end-to-end solution provider for the pharmaceutical and biotech industry, is excited to announce the opening of its new subsidiary, ZETA PL Sp. z o.o., in Gdansk, Poland.

Rockwell Automation (NYSE: ROK), the world’s largest company dedicated to industrial automation and digital transformation, today announced a strategic collaboration with ZETA, a global end-to-end solution provider for the pharma and biotech industry.

ZETA is thrilled to announce that it has recently signed contracts for the acquisition of shares in the Swiss company CB Consultancy. As part of this agreement, ZETA acquired a 20% share in CB Consultancy, the deal has been closed by 14 July 2023.

The biopharmaceutical market in the Asian region shows enormous growth potential. The internationally active ZETA Group has established itself as an end-to-end solution provider for the pharmaceutical and biotech industries in this dynamic environment. With its majority interest in the Indian engineering and plant construction specialist Biotree, ZETA has intensified its development of the Asian market. Local companies benefit from the high quality of their service and performance portfolio.

Blood plasma forms the basis of many life-saving therapies and is also an important building block for future active ingredients in medical research. As the general planner for Octapharma, ZETA implemented an industry-leading EPCM project resulting in the doubling of plasma throughput at the Vienna, Austria, manufacturing site.

Swiss Bühler Group today announces the formation of a new joint venture with Austrian pharmaceutical and biotechnology company ZETA. The new company, Eridia, will engineer food and feed biotechnology plants primarily in the fields of precision fermentation and cellular agriculture.

The pharmaceutical industry continues to grow rapidly due to increasing demands for innovative process solutions, full-scale automation, and new manufacturing technologies. To meet this increasing market demand, ZETA established its North American engineering hub in Greater Philadelphia in August 2021, providing ultra-fast-track project delivery.

ZETA has established itself internationally as a one-stop-shop for the biopharmaceutical industry. With ZETA's opening of its new subsidiary in Singapore, Asian companies in the pharmaceutical and biotech industries can now also benefit from customer-specific services on-site.

ZETA’s many years of experience in the management of major projects in the biopharmaceutical industry coupled with the sophisticated software solutions from SIEMENS recently inspired the two companies to enter into a strategic partnership to drive the digital transformation of the pharmaceutical sector.
Acting as a one-stop-shop for its customers in the pharmaceutical and biotechnology industries and meeting all their requirements is a top priority for ZETA.

ZETA delivers integrated engineering solutions for the pharmaceutical and biotech industries. The services span the entire project lifecycle—from early development and design to turnkey delivery and long-term operational support.

ZETA delivers tailored automation solutions that enable pharmaceutical and biotech manufacturers to scale, adapt, and innovate.

ZETA is a trusted partner in designing and delivering cutting-edge facilities across the full spectrum of pharmaceutical and biotech modalities.

ZETA delivers high-performance EPCMV (Engineering, Procurement, Construction Management, and Validation) services for pharmaceutical and biotech facilities.

ZETA designs and delivers customised skid systems and process equipment for pharmaceutical and biotech manufacturing.

ZETA integrates process simulation at the core of pharmaceutical and biotech engineering.

ZETA’s digital solutions enable pharmaceutical and biotech companies to accelerate innovation, streamline operations, and unlock new levels of efficiency.

ZETA’s magnetic agitators are engineered to meet the highest standards in aseptic processing. Leveraging decades of engineering experience and close collaboration with end users, ZETA delivers mixing solutions that combine contamination control, process reliability, and operational efficiency.
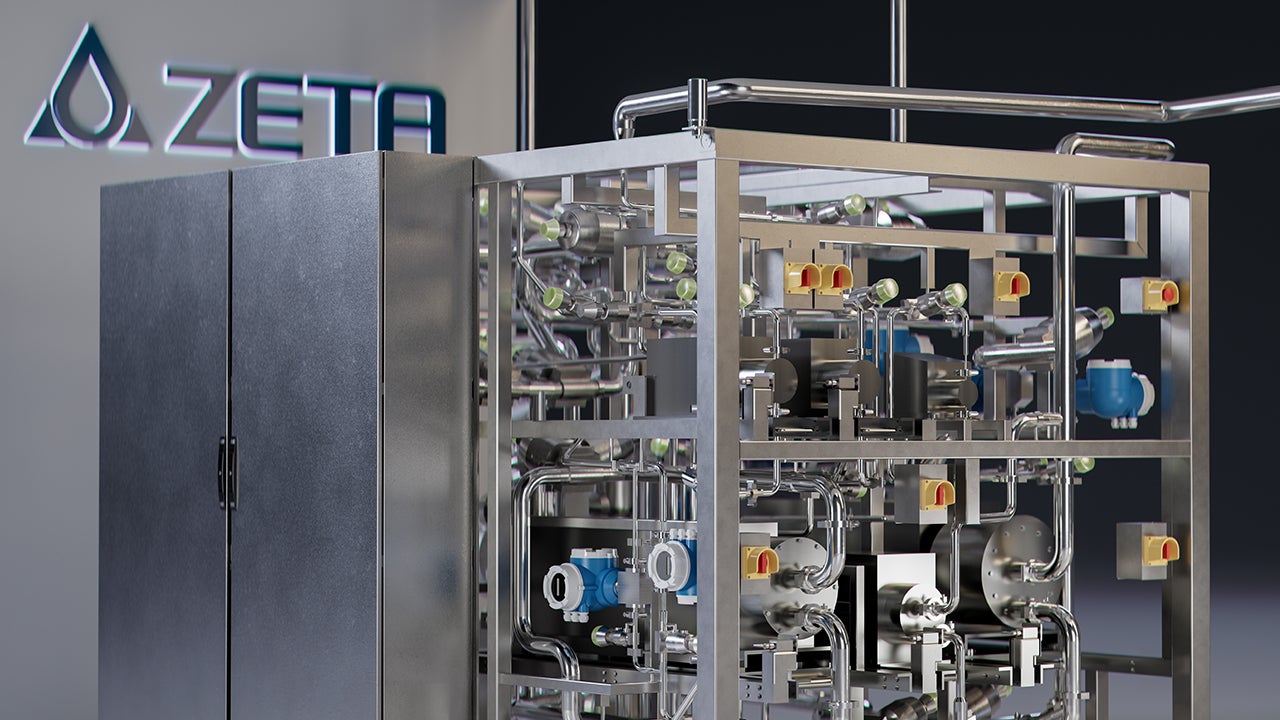
ZETA’s InFlow system transforms buffer preparation by enabling just-in-time, on-demand generation directly at the point of use.

ZETA boasts modern pharmaceutical production facilities with deep professional expertise and unwavering commitment to quality.