SCHRADER specialises in high-quality technologies for the extraction of high-purity active ingredients for the pharmaceutical industry.
The company engages in the design and development of extraction plants both with and without downstream evaporation, distillation or rectification, and plants for preparation and mixing technology.
Extraction technologies for high-purity active pharmaceutical ingredients
Processes used in the pharmaceutical industry derive from the same technologies, and the company offers customisation to specific client requirements through to turnkey plant creation.
The pharmaceutical industry demands the highest levels of diligence, reproducibility and quality. SCHRADER attaches particular significance to qualification and the development and production in compliance with standards.
Complete documentation based upon Good Manufacturing Practice (GMP) and the Food and Drug Administration (FDA) guidelines is imperative.
SCHRADER carries out the following qualification and validation activities for plants in line with Annex 15 of the European Union (EU) GMP guidelines: performance qualification (PQ), operational qualification (OQ), installation qualification (IQ), factory acceptance test (FAT) and design qualification (DQ).
Extraction technologies for the pharmaceutical industry
Precise dosing and mixing are necessary to produce homogeneous products of consistent quality in the pharmaceutical industry.
SCHRADER ensures efficient and reliable preparation and mixing technology by designing suitable agitator vessels.
Turnkey plants ensure verifiable and safe production made to measure, from implementing the recipe instructions to the monitoring and documentation of all parameters.
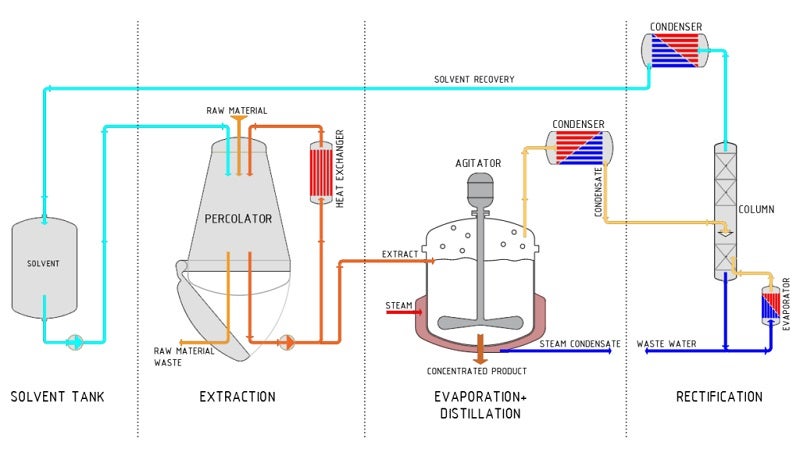
The initial stage of the process workflow is extraction. The type of product extracted and the quantity of raw material determines the choice of the extraction process.
Procedures such as evaporation, distillation and rectification can be integrated into the plant concept as required. Other techniques include mixing, dissolving, sterilising, cleaning, homogenisation and roasting and upstream methods such as grinding, chopping and swelling.
Pharmaceutical extraction technologies to exceed expectations
SCHRADER manufactures individual and highly specialised systems according to client specifications and in line with the relevant regulations.
Based on objectives, process overview and the evaluated parameters, SCHRADER develops and documents a plant concept optimal in terms of energy efficiency, sustainability and process safety.
SCHRADER’S goal is to achieve high-quality end products with high production reliability to satisfy its clients.
Extraction of pharmaceutical ingredients
SCHRADER engages in the extraction of ingredients from medicinal plants for the production of pharmaceuticals. Many herbal medicines contain only dried plant constituents or extracts.
A complex, multi-stage extraction and purification process is required to produce a constituent, in the course of which the desired parts become enriched and undesired ingredients removed. The composition and quantity of these components become standardised to ensure consistent quality.
Quality, flexibility and technical competence
SCHRADER places significance on inspections, servicing and maintenance to reduce the frequency of malfunctions and equipment failures in client production.
Should obstacles transpire, SCHRADER’s specially trained service engineers will be on hand with the essential equipment as quickly as possible to carry out servicing and maintenance.
SCHRADER will be at its client’s disposal as a contact partner for continuous optimisation and plant service, even after plant completion and commissioning.