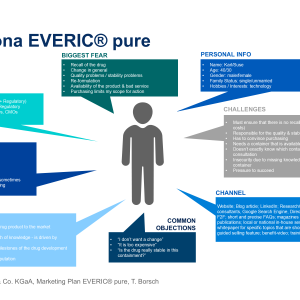
SCHOTT Launches New Pharmaceutical Vials with Reduced Delamination Risk
SCHOTT is to launch SCHOTT Vials DC, which are pharmaceutical vials that, for the first time ever, allows for the risk of delamination to be determined based on threshold values.
SCHOTT monitors these values over the course of the manufacturing process and is thus able to minimize the risk of delamination. The company succeeded in optimising its manufacturing process to ensure that SCHOTT Vials DC have a more homogeneous surface, hence offering high chemical stability.
Furthermore, SCHOTT is the first manufacturer to develop a patented test method that even allows for this lower tendency to delamination to be documented – known as SCHOTT Delamination Quicktest. SCHOTT Vials DC will be available as 2R to 10R ISO vials starting at the beginning of 2014.
The problem of delamination, in other words the peeling off of flakes from the inner glass surface of a pharmaceutical vial as a result of interaction with the formulation and / or medication, has become increasingly important to the pharmaceutical industry in recent years. Numerous recalls clearly confirm this, and the US drug authority in turn is explicitly requiring that pharmaceutical companies manage their risks more closely.
SCHOTT Vials DC thus represents an interesting solution for pharmaceutical companies interested in lowering the risk of delamination by selecting an improved packaging product. These vials are an interesting alternative not only for new products, but also for products that are already well- established in the marketplace.