The pharmaceutical industry continues to be a hotbed of innovation, with activity driven by the evolution of new treatment paradigms, and gravity of unmet need, as well as the growing importance of technologies such as pharmacogenomics, digital therapeutics, and artificial intelligence. In the last three years alone, there have been over 633,000 patents filed and granted in the pharmaceutical industry, according to GlobalData’s report on Innovation in Pharmaceuticals: Adeno-associated virus vectors. Buy the report here.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
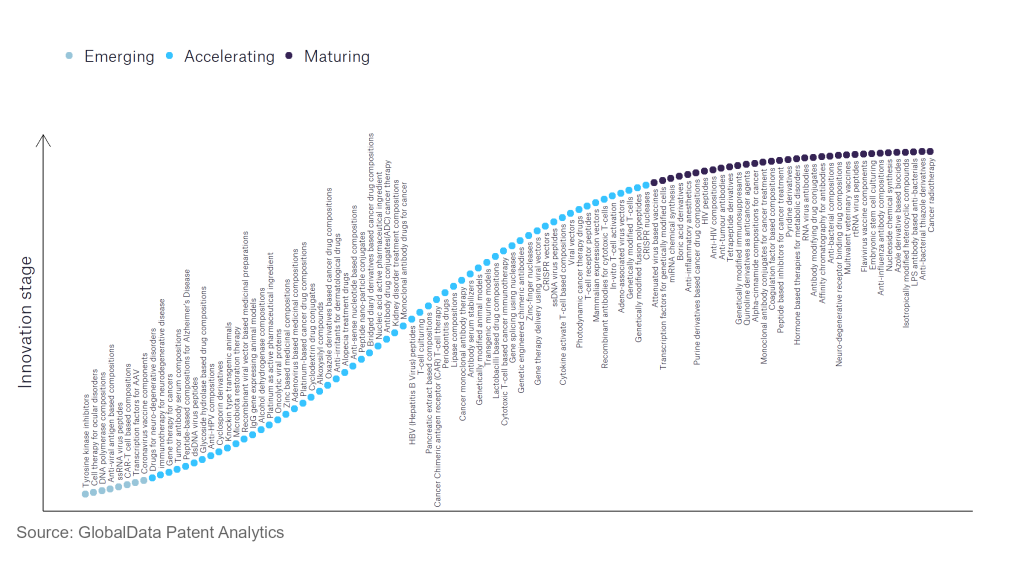
However, not all innovations are equal and nor do they follow a constant upward trend. Instead, their evolution takes the form of an S-shaped curve that reflects their typical lifecycle from early emergence to accelerating adoption, before finally stabilising and reaching maturity.
Identifying where a particular innovation is on this journey, especially those that are in the emerging and accelerating stages, is essential for understanding their current level of adoption and the likely future trajectory and impact they will have.
110 innovations will shape the pharmaceutical industry
According to GlobalData’s Technology Foresights, which plots the S-curve for the pharmaceutical industry using innovation intensity models built on over 756,000 patents, there are 110 innovation areas that will shape the future of the industry.
Within the emerging innovation stage, cell therapy for ocular disorders, coronavirus vaccine components, and DNA polymerase compositions are disruptive technologies that are in the early stages of application and should be tracked closely. Adeno-associated virus vectors, alcohol dehydrogenase compositions, and antibody serum stabilisers are some of the accelerating innovation areas, where adoption has been steadily increasing. Among maturing innovation areas are anti-influenza antibody compositions and anti-interleukin-1, which are now well established in the industry.
Innovation S-curve for the pharmaceutical industry
Adeno-associated virus vectors is a key innovation area in pharmaceutical
Adeno-associated viruses (AAVs) are a sub-category of DNA virus vectors, modified viruses that act as transport vehicles to deliver genetic material into target cells for gene therapy. AAVs are small single stranded DNA viruses that are non-pathogenic to humans and are commonly used for gene delivery in vivo because of their mild immunogenicity. AAVs can infect both dividing and quiescent cells. They can direct long-term transgene expression, but generally are not permanently integrated into the host genome and are limited by their small packaging capacity. The first two gene therapy products approved by the FDA used AAV2 and AAV9 vectors for gene delivery.
GlobalData’s analysis also uncovers the companies at the forefront of each innovation area and assesses the potential reach and impact of their patenting activity across different applications and geographies. According to GlobalData, there are 270+ companies, spanning technology vendors, established pharmaceutical companies, and up-and-coming start-ups engaged in the development and application of AAV vectors.
Key players in adeno-associated virus vectors – a disruptive innovation in the pharmaceutical industry
‘Application diversity’ measures the number of different applications identified for each relevant patent and broadly splits companies into either ‘niche’ or ‘diversified’ innovators.
‘Geographic reach’ refers to the number of different countries each relevant patent is registered in and reflects the breadth of geographic application intended, ranging from ‘global’ to ‘local’.
Voyager Therapeutics is one of the leading patent filers for AAV vectors. The company is evaluating its AAV-based drugs for various central nervous system (CNS) disorders, genetic disorders and oncology indications. The company has entered into separate agreements with Novartis and Pfizer for the potential use of its drugs in CNS indications.
Another leading filer is UniQure, which is developing AAV-based gene therapies using its gene technology platform and offers disease-modifying treatments to patients with severe genetic diseases and other diseases. The company’s AAV programmes are focused on haematological disorders, CNS disorders, and cardiovascular diseases. The company entered into a licensing agreement with CSL for exclusive global rights to etranacogene dezaparvovec, UniQure’s investigational gene therapy for patients with haemophilia B.
To further understand the key themes and technologies disrupting the pharmaceutical industry, access GlobalData’s latest thematic research report on Pharmaceutical.
Data Insights
From

The gold standard of business intelligence.
Blending expert knowledge with cutting-edge technology, GlobalData’s unrivalled proprietary data will enable you to decode what’s happening in your market. You can make better informed decisions and gain a future-proof advantage over your competitors.



