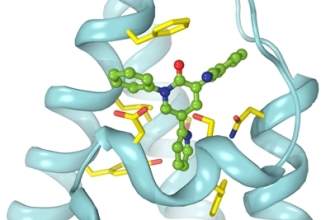

US researchers worked to develop new Epilepsy drugs
Researchers at Columbia University Medical Centre (CUMC) identified how anti-epileptic drug Perampanel blocks AMPA receptors in the brain that help in the transmission of electrical signals and play a role in the development of seizures.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Epilepsy is a group of neurological diseases characterised by epileptic seizures.
An improved understanding of how epilepsy drugs work could lead to the development of more effective drugs with fewer side effects.
The scientists studied the effects of Perampanel on rat AMPA receptors, which are almost similar to human receptors.
GE planned to invest €150 million for biopharmaceutical manufacturing campus in Ireland

GE planned to invest €150 million in a new biopharmaceutical manufacturing campus on Industrial Development Agency's site (IDA) at Loughbeg, Ringaskiddy, County Cork, Ireland.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataGE BioPark Cork is subject to contract and planning approvals and will feature Europe’s first KUBio, a prefabricated, off-the-shelf bio-manufacturing facilities, owned and operated by GE customers.
This site will serve as a focal point for further investment in next-generation Irish biopharmaceutical manufacturing.
Once the campus becomes fully operational, it is expected to provide more than 500 jobs, approximately 400 with biopharma companies and another 100 directly by GE.
Scientists found new immunotherapy effective against pancreatic cancer

Scientists at St George’s, University of London found that a new immunotherapy treatment could increase the life expectancy of pancreatic cancer patients.
The IMM-101 drug, along with chemotherapy, was trialled on patients with advanced pancreatic cancer.
As part of the trial, one group of patients were given gemcitabine chemotherapy through a drip, as well as a course of IMM-101 injections, while the other group received gemcitabine chemotherapy alone.
Some patients who were given both treatments lived significantly longer than expected, while the overall median survival increased by 59%, according to the study.
WHO declared Sri Lanka malaria-free

The World Health Organisation (WHO) certified Sri Lanka for eliminating malaria disease, which long affected the island country.
Between the 1970s and 1980s, malaria cases in Sri Lanka increased, following which the country’s anti-malaria campaign introduced a new strategy in the 1990s to target the parasite in addition to targeting the mosquito.
WHO regional director Dr Poonam Khetrapal Singh said: “Sri Lanka’s achievement is truly remarkable. In the mid-20th century it was among the most malaria-affected countries, but now it is malaria-free.
“This is testament to the courage and vision of its leaders, and signifies the great leaps that can be made when targeted action is taken.”
Parexel entered definitive agreement to acquire ExecuPharm
US-based Parexel, one of the world's biggest contract research organisations, entered a definitive agreement to purchase ExecuPharm for an undisclosed amount.
Headquartered in the US and established in 1995, ExecuPharm is a functional service provider (FSP) catering to the biopharmaceutical industry.
It provides qualified professionals to cater to clients across functional areas such as clinical monitoring or study management along with other operational activities, including onboarding, training, line management, performance management and policy administration.
US FDA granted approval for Novartis' Ilaris to treat Periodic Fever Syndromes
Novartis received the US Food and Drug Administration's (FDA) approval for Ilaris (canakinumab) to treat rare Periodic Fever Syndrome conditions.
Periodic Fever Syndromes are a group of diseases that cause serious recurrent fever and pathogenic inflammation through non-infectious activation of the immune system.
Ilaris inhibits Interleukin-1 (IL-1) beta, which is an important part of the body's immune system defences. Excessive production of IL-1 beta causes certain inflammatory diseases.
This human monoclonal antibody hampers IL-1 beta's activity for a sustained period of time, therefore inhibiting inflammation that is caused by its over-production.
Allele received NIH grant to develop new antibody therapy for Alzheimer’s disease
Allele Biotechnology and Pharmaceuticals (Allele) secured a grant from the US National Institutes of Health's (NIH) National Institute on Aging to develop a new antibody therapy for Alzheimer’s disease.
Allele seeks to use the NIH grant to develop antibody drug candidates to combat, as well as provide, long-needed research tools for other scientists to further studies on Alzheimer’s disease.
In an earlier study, Allele scientists and academics collaborators discovered a strong correlation between a previously uncharacterised target gene and Alzheimer’s disease.
It was observed that the expression of the gene decreases amyloid beta production and tau phosphorylation, which are the components responsible for plaque formation in Alzheimer’s disease.
Kenya launched world's first child-friendly TB medicines
Kenya launched the world's first drug specifically designed to treat tuberculosis (TB) in children.
The launch follows a recent prequalification by the World Health Organisation to use improved child-friendly medicines for TB treatment in children.
The latest flavoured medicines must be dissolved in water before being administered to children, Kenya Ministry of Health said in a statement.
Medical services director Dr Jackson Kioko said: “Until recently, the child TB treatment regimen comprised of multiple pills of many formulations. This regimen was complex to use for both healthcare workers and caregivers.
UK’s NICE recommended Bayer’s Eylea for adults with visual impairment
The UK National Institute for Health and Care Excellence (NICE) recommended Bayer’s Eylea (aflibercept solution for injection) as a first-line treatment option for adults with visual impairment due to macular oedema, secondary to branch vein occlusion (BRVO).
A blood clot in one of the branches of the retina's main vein in the retina causes BRVO.
Due to blockages in the retinal veins, the pressure in the small blood vessels at the back of the eye increases and leads to blood and fluid leaking beneath the macula.
If not treated well, macular oedema can impact a person’s vision.
GSK planned to sell remaining stake in South African drugmaker Aspen
GlaxoSmithKline (GSK) planned to divest its remaining stake in South African drugmaker Aspen Pharmacare.
The company aimed to sell up to 28.2 million ordinary shares in Aspen for a price that will be determined by means of an accelerated bookbuild offering process.
The potential sale represents about 6.2% of the issued share capital.
GSK was a shareholder in Aspen for seven years and will use the proceeds from the transaction for general corporate purposes.
GSK signed a placing agreement with Citigroup Global Markets and UBS, in a bid to act as joint book-runners for the offering.
AssistRx and NewCrop collaborated to improve capabilities for electronic health records
US-based provider of web-based pharmaceutical data management solutions AssistRx collaborated with local e-prescribing service firm NewCrop to improve capabilities for electronic health records (EHRs) and healthcare organisations.
Throughout the collaboration, NewCrop will have the ability to offer its clients AssistRx's patient-focused software solution, iAssist Workflow.
The HIPAA-compliant, secure, cloud-based software is said to eliminate paper and streamline communication between prescribers, pharmacies and manufacturers, thereby reducing the time taken by the patients to receive their speciality drugs.




