There were 256 deals recorded involving top pharmaceutical companies in the three months to April with a number of high profile contract service agreement, licensing agreement, partnership, merger, venture financing, equity offering, asset transaction, debt offering, acquisition and private equity deals.
That’s according to GlobalData’s Financial Deals database, which tracks market activity across a variety of sectors and deal types.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The deals below only include those that have been completed – so excludes rumours or those that have been agreed but not yet executed.
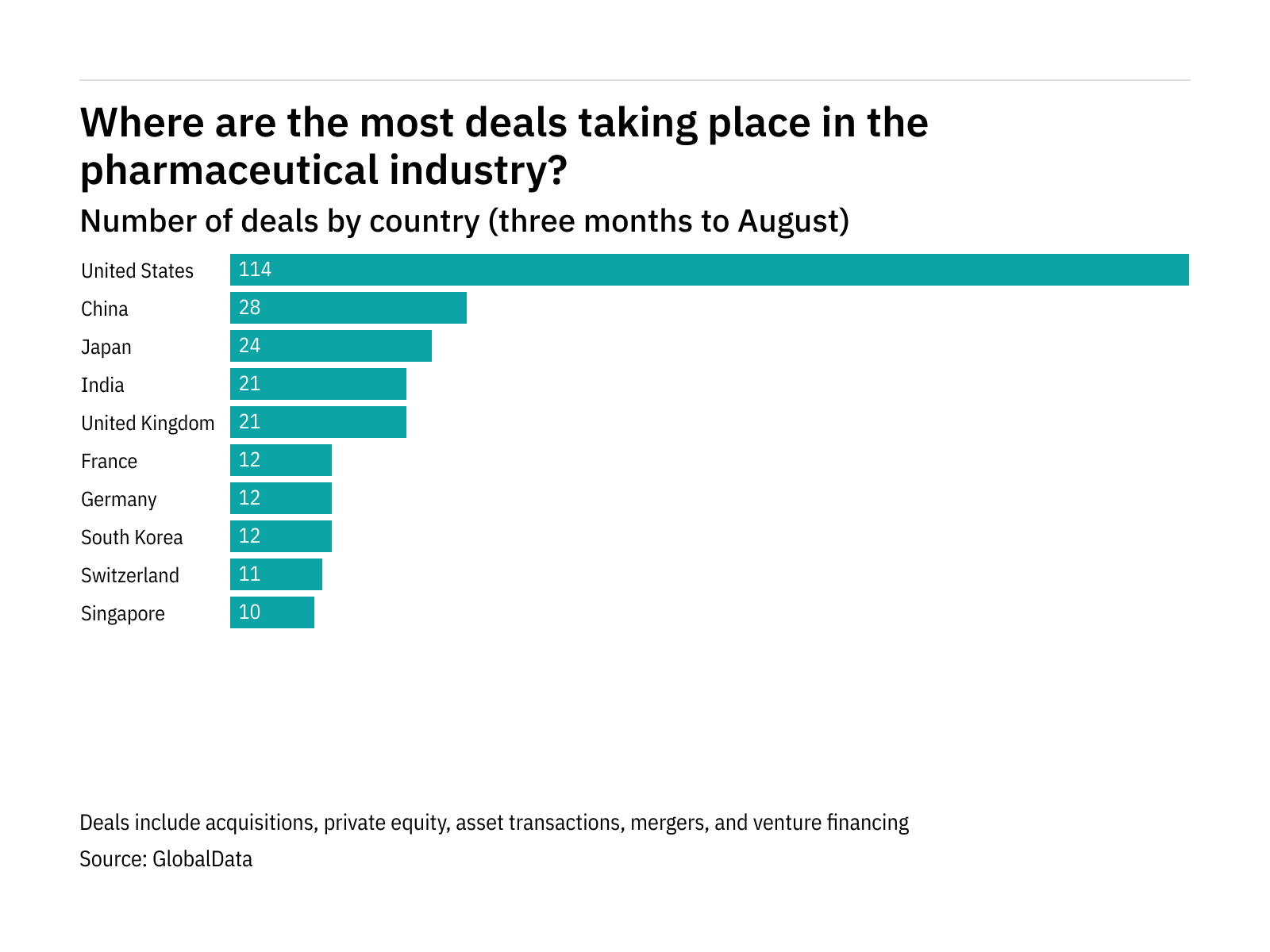
The figures, which cover the top pharmaceutical companies, show the market in the US to be the most active, with 114 deals taking place over the last three months. That was followed by China, which saw 28 deals.
Below are some of the largest completed deals to have taken place in the last quarter.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataAcquisitions
GlaxoSmithKline to Acquire Sierra Oncology - 12 April ($1,900m)
GlaxoSmithKline Plc, has entered into an agreement to acquire Sierra Oncology Inc, a biopharmaceutical company focused on targeted therapies for the treatment of rare forms of cancer, for $55 per share of common stock in cash representing an approximate total equity value of $1.9 billion (£1.5 billion).
Under the terms of the agreement, the acquisition will be effected through a one-step merger in which the shares of Sierra Oncology outstanding will be cancelled and converted into the right to receive $55 per share in cash.
The per share price represents a premium of approximately 39% to Sierra Oncology's closing stock price on 12 April 2022 and a premium of approximately 63% to Sierra's volume-weighted average price (VWAP) over the last 30 trading days. Sierra Oncology's Board of Directors has unanimously recommended that Sierra's stockholders vote in favour of the approval of the merger. Additionally, stockholders of Sierra Oncology holding approximately 28% of Sierra's outstanding shares, have agreed to vote their shares in favour of approval of the merger.
GSK will account for the transaction as a business combination and expects it to be accretive to adjusted EPS in 2024, the expected first full year of momelotinib's sales. The value of the gross assets of Sierra Oncology to be acquired (as of 31 December 2021) is $109 million. The net losses of the business were $95 million for the 12 months ended 31 December 2021 (£70 million, at the rate of £1 = $1.38, being the average rate for the period).
PJT Partners is acting as financial advisor and Cleary Gottlieb Steen & Hamilton LLP is serving as legal counsel to GSK. While, Lazard is acting as financial advisor and Wilson Sonsini Goodrich & Rosati is serving as legal counsel to Sierra Oncology in connection with the transaction.
Subject to customary conditions, including the approval of the merger by at least a majority of the issued and outstanding shares of sierra oncology, and the expiration or earlier termination of the waiting period under the Hart-Scott-Rodino Antitrust Improvements Act of 1976, the transaction is expected to close in the third quarter of 2022 or before.
Biohaven Pharma to Acquire Channel Biosciences from Knopp Biosciences - 25 February ($1,238m)
Biohaven Pharmaceutical Holding Company, a commercial-stage biopharmaceutical company with a portfolio of therapies in neurological and neuropsychiatric diseases, announced it would acquire Channel Biosciences a subsidiary of Knopp Biosciences LLC and its Kv7 channel targeting platform – adding the latest advances in ion-channel modulation to Biohaven's growing neuroscience portfolio.
In consideration for the transaction, Biohaven will make an upfront payment comprised of $65 million in Biohaven common shares and $35 million in cash to Knopp Biosciences. Biohaven has also agreed to make additional success-based earnout payments (i) up to $325 million based on BHV-7000 developmental and regulatory epilepsy milestones through approvals in the US, EU and Japan, (ii) up to an additional $250 million based on developmental and regulatory milestones for the Kv7 pipeline development in other indications and additional country approvals and (iii) up to $562.5 million in scaled, commercial annual sales-based milestones of BHV-7000, the total of which will be achieved when annual sales exceed USD3 billion. Biohaven has also agreed to make scaled royalty payments for BHV-7000 and the pipeline programs, starting at high single digits and peaking at low teens for BHV-7000 and starting at mid-single digits for the pipeline programs.
The acquisition of BHV-7000 and the Kv7 channel-targeting series demonstrates Biohaven's continued commitment to neurology and to meeting the unmet needs of these patients. Biohaven intends to bring BHV-7000 to the clinic in 2022, with focal epilepsy as the lead indication for development.
AbbVie Acquires Syndesi Therapeutics - 01 March ($1,000m)
AbbVie has acquired Syndesi Therapeutics SA, a clinical stage biotechnology company and a portfolio company of Novo Holdings.
The acquisition gives AbbVie access to Syndesi's portfolio of novel modulators of the synaptic vesicle protein 2A (SV2A), including its lead molecule SDI-118.
AbbVie will pay Syndesi shareholders a $130 million upfront payment with the potential for Syndesi shareholders to receive additional contingent payments of up to USD870 million based on the achievement of certain predetermined milestones.
The acquisition strengthens AbbVie's neuroscience portfolio.
Cleary Gottlieb Steen & Hamilton LLP acted as legal counsel to AbbVie. Goodwin Procter LLP acted as lead legal counsel, along with Deloitte Legal, Belgium, and Lazard acted as the exclusive financial adviser to Syndesi.
Halozyme Therapeutics to Acquire Antares Pharma - 13 April ($960m)
Halozyme Therapeutics, has entered into a definitive agreement pursuant to acquire Antares Pharma for $5.60 per share in cash.
The transaction, which values Antares at approximately $960 million, was unanimously approved by both the Halozyme and Antares Boards of Directors.
On April 26, 2022, Halozyme Therapeutics announced that it is commencing, through a wholly owned subsidiary, Atlas Merger Sub (Purchaser), a cash tender offer to purchase all outstanding shares of common stock of Antares Pharma, for $5.60 per share in cash.
Under the terms of the merger agreement, Halozyme will commence a cash tender offer to acquire all of the outstanding shares of Antares for $5.6 per share in cash. The transaction is not subject to a financing condition. Halozyme intends to finance the transaction using existing cash on hand and new sources of debt. Following completion of the transaction, Halozyme expects to maintain a strong balance sheet with less than 3.5x net debt-to-EBITDA ratio at the time of transaction close. Net debt-to-EBITDA ratio is expected to decline significantly in the quarters post transaction close. The closing of the tender offer will be subject to certain conditions, including the tender of shares representing at least a majority of the total number of Antares' outstanding shares of common stock, the expiration or termination of the HSR waiting period, and other customary conditions. Following the successful completion of the tender offer, Halozyme will acquire all remaining shares not tendered in the tender offer through a second-step merger at the same price.
The transaction is expected to be immediately accretive to Halozyme's 2022 revenue and non-GAAP earnings and to accelerate top- and bottom-line growth through 2027, with multiple growth drivers beyond 2027. The combination of Halozyme and Antares will create a leading drug delivery and specialty product company. The Antares business consists of a best-in-class, differentiated, royalty revenue generating auto injector platform business that offers broad licensing opportunity, and a commercial business, with three proprietary commercial products.
BofA Securities and Wells Fargo Securities LLC are acting as financial advisors to Halozyme and Weil, Gotshal & Manges LLP is acting as legal advisor. While, Jefferies LLC is acting as financial advisor and Skadden, Arps, Slate, Meagher & Flom LLP is acting as legal advisor to Antares in connection with the transaction.
The tender offer is scheduled to expire on May 23, 2022, unless extended in accordance with the terms of the Merger Agreement. The closing of the tender offer is subject to certain conditions, including the tender of shares representing at least a majority of the total number of Antares' outstanding shares of common stock, the expiration or termination of the HSR waiting period, and other customary conditions. The transaction is expected to close in the first half of 2022.
Mergers
Ligand Pharma to Merge with Avista Public Acquisition - 23 March
Ligand Pharmaceuticals Incorporated (Ligand) has announced the signing of a definitive merger agreement with Avista Public Acquisition Corp. II (APAC), a publicly traded special purpose acquisition company (SPAC), providing for the spin-off of OmniAb, Inc.
The combined company will be led by Ligand's President, Matt Foehr, and will be renamed “OmniAb, Inc.”
Upon the closing of the transaction, Avista Capital Partners (Avista), APAC's sponsor and a leading private equity firm focused on the healthcare industry, has agreed to invest up to $115 million in the combined company, and Ligand will contribute $15 million. The combined company will have an initial pre-money equity valuation of $850 million. Immediately prior to the transaction close, Ligand intends to distribute 100% of its ownership of OmniAb to Ligand shareholders in a tax-free distribution.
Ligand's OmniAb antibody discovery platform provides pharmaceutical industry partners with access to diverse antibody repertoires and high-throughput screening technologies to enable discovery of next-generation therapeutics.
The combination of OmniAb and AHPA is structured to guarantee a minimum of $130 million in gross cash to the combined company at the time of closing, and up to $266 million in the event of no redemptions by APAC shareholders. APAC's shareholders will be eligible to participate in the transaction or to elect redemption of their shares. Avista has agreed to guarantee that Avista and AHPA will provide at least $115 million of gross cash to the combined company through a $15 million PIPE investment and a USD100 million facility to backstop potential redemptions. Ligand's $15 million contribution to OmniAb will be made irrespective of the number of redemptions or the Avista contributions.
Ligand intends to distribute 100% of the equity in OmniAb to Ligand shareholders immediately prior to the business combination with APAC. The transaction will be effected through a “Reverse Morris Trust” transaction pursuant to which OmniAb will be spun-off to Ligand's shareholders and simultaneously merged as a subsidiary of APAC. The transaction is expected to be tax-free to Ligand and its shareholders for US federal income tax purposes, except for cash received in lieu of fractional shares. Upon the closing of the transaction, Ligand shareholders are expected to own approximately 75% to 84% of the combined company, depending on redemptions, which will be listed on the Nasdaq Global Markets under the ticker symbol “OABI”.
Credit Suisse is acting as lead capital markets and financial advisor to OmniAb, Cowen, Stifel, SVB Leerink and Truist Securities are also acting as capital markets and financial advisors to OmniAb, and CJS Securities, Craig-Hallum Capital Group, H.C. Wainwright & Co. And Roth Capital Partners are acting as advisors to OmniAb. Weil, Gotshal & Manges LLP is legal advisor to APAC. Latham & Watkins LLP is legal advisor to Ligand.
The Boards of Directors of both APAC and Ligand have unanimously approved the proposed transaction, which is subject to customary closing conditions, including receipt of required regulatory approvals and receipt of approval from APAC's shareholders.
Venture financing
LifeMine Therapeutics Raises $175 Million in Series C Financing - 23 March ($175m)
LifeMine Therapeutics Inc., a biopharmaceutical company reinventing drug discovery by mining genetically-encoded small molecules (GEMs) from the biosphere, announced the completion of a $175 million Series C financing. The financing was led by new investor Fidelity Management & Research Company, with participation by additional new investors Invus and 3W Partners Capital; GlaxoSmithKline (GSK) also joined the round as a strategic partner. Existing investors, including GV, Arch Venture Partners, Blue Pool Capital and MRL Ventures Fund, also participated.
Concurrently, LifeMine Therapeutics announced a R&D collaboration with GSK, which will provide GSK with access to LifeMine's genomically enabled drug discovery platform to identify novel small molecule leads directed to up to three human targets provided by GSK addressing multiple disease areas.
Proceeds from the financing will be used to advance LifeMine's proprietary, evolutionarily-derived genomic drug discovery platform, Avatar-Rx.
Goodwin Procter LLP acted as legal advisor to the company for the financing.
Be Biopharma Raises $130 Million in Series B Financing - 14 April ($130m)
Be Biopharma Inc, a biopharmaceutical company engaged in the discovery and development of Engineered B Cell Medicines (BeCM), has raised $130 million in series B financing. The financing was led by ARCH Venture Partners and joined by Bristol Myers Squibb, Atlas Venture, RA Capital Management, Alta Partners, Longwood Fund and Takeda Ventures.
The company intends to use the funds to advance it's proprietary autologous and allogeneic BeCM platforms across multiple therapeutic areas and progress pipeline candidates toward the clinic.
Octant Raises $80 Million in Series B Financing - 21 April ($80m)
Octant, Inc., a synthetic biology drug discovery company designing small molecule, multi-target drug leads for multifactorial diseases, has raised $80 million in series B financing. The financing was led by Catalio Capital Management, with participation from Bristol Myers Squibb, and from existing investors Andreessen Horowitz Bio Fund, Allen & Co., and 50 Years VC.
This latest round brings Octant total funding raised to $115 million.
Concurrently, Octant announced a collaboration with Bristol Myers Squibb. Under the collaboration agreement, Octant will apply its DMS technologies to a set of inflammation-related pathways.
The company intends to use the proceeds to further expand Octant's platform capabilities and pipeline, advance its proprietary drug discovery technology, and generate extensive datasets that map the relationships between drug candidates, genetics, and the biochemical mechanisms of human cells.
Ashvattha Therapeutics Raises $69 Million in Series B Financing - 27 April ($69m)
Ashvattha Therapeutics, a developer of novel hydroxyl dendrimer therapeutics, has raised $69 million in series B financing led by Huadong Medicine Investment Holding Co Ltd with participation from existing investors, including Natural Capital Investment Fund, Plum Alley Investments and Tribe Capital and several angel investors.
Arkuda Therapeutics Raises $64 Million in Series B Financing - 10 February ($64m)
Arkuda Therapeutics, a company engaged in developing medicines to change the trajectory of neurodegenerative disease, has raised $64 million in series B financing. The finance was co-led by Cormorant Asset Management and Pivotal bioVenture Partners, with participation from Surveyor Capital (a Citadel company), and Eli Lilly and Company and with participation from existing investors Atlas Venture, Pfizer Ventures, Tekla Capital Management LLC and Mission BioCapital.
The company intends to use the proceeds to support progression of lead progranulin enhancer program into IND-enabling studies.
Private equity
Cinven to Acquire Environmental Science Professional Business from Bayer - 10 March ($2,600m)
Cinven Ltd, a UK-based private equity firm providing funding and investment services, has entered into an agreement to acquire Environmental Science Professional business from Bayer AG, a Germany-based pharmaceutical and life sciences company, for a purchase consideration of $2.6 billion.
Bayer's Environmental Science Professional Business provides solutions to control pests, disease and weeds in non-agricultural areas such as vector control, professional pest management, industrial vegetation management, forestry, and turf and ornamentals.
Hengeler Mueller is acting as legal advisor to Bayer.
BofA Securities is acting as financial advisor to Bayer.
Blackstone Life Sciences to Invest up to $328.3 Million in Sanofi - 15 March ($328m)
Funds managed by Blackstone Life Sciences, an affiliate of The Blackstone Group Inc., a private equity firm, has announced to invest up to EUR300 million (USD328.3 million) in Sanofi.
Sanofi will continue to fully manage the clinical program and retain full rights and control of Sarclisa (isatuximab).
The company intends to use the proceeds to accelerate the global pivotal studies and the clinical development program for the subcutaneous formulation and delivery of the anti-CD38 antibody Sarclisa, to treat patients with multiple myeloma (MM).
Goodwin Procter is acting as legal counsel to Blackstone Life Sciences in connection with the transaction.
If successful, BXLS will be eligible to receive royalties on future subcutaneous sales. The pivotal study for the subcutaneous formulation is expected to begin in the second half of 2022.
Asset transactions
Biocon Biologics to Acquire Biosimilars Business from Viatris - 27 February ($3,335m)
Biocon Biologics Ltd. (BBL), has agreed to acquire Viatris' biosimilars business from Viatris Inc. Viatris will receive consideration of up to $3.335 billion in cash and stock.
BBL currently has a portfolio of 20 biosimilars. The acquisition of biosimilars assets of Viatris significantly strengthens BBL's position in providing affordable access to patients through its portfolio in diabetes, oncology, immunology and other non-communicable diseases.
Viatris will receive cash consideration of $2 billion on closing of the transaction and up to $335 million as additional payments expected to be paid in 2024. Additionally, upon closing of the transaction, BBL will issue $1 billion of Compulsorily Convertible Preference Shares (CCPS) to Viatris, equivalent to an equity stake of at least 12.9% in the company, on a fully diluted basis.
The companies will also enter into a Transition Services Agreement, pursuant to which Viatris will provide certain transition services, including commercialization services, for an expected two-year period. Viatris also will pay USD50 million to BBL to fund certain capital expenditures.
BBL acquires: (i) Viatris' global commercial infrastructure in developed and emerging markets, (ii) Viatris' global biosimilars business with an estimated revenue of $875 million and EBITDA of USD200 million for CY 2022 and estimated to exceed $1 billion in revenue next year, (iii) Viatris' rights in all biosimilars assets including its in-licensed portfolio and an option to acquire Viatris' rights in bAflibercept, and (iv) Transition services for an expected two-year period to ensure a seamless transition with partners and continued services to patients and customers.
The revenues of this acquired business are estimated to be $1 billion next year.
The cash payment of $2 billion to be funded by $800 million raised through equity infusion in BBL and the remainder to be funded by debt, additional equity or a combination thereof. BBL has received expressions of interest from financial institutions for debt financing and equity commitments from existing shareholders.
The transaction will enable Biocon Biologics' to create a unique fully integrated global biosimilars enterprise.
Allegro Capital served as the financial advisor to BBL. Goodwin Procter and Shardul Amarchand Mangaldas served as BBL's legal advisors to this transaction.
The transaction is expected to close in H2-2022, will be value accretive to Biocon and BBL shareholders.
BioMarin Pharma to Sell Rare Pediatric Disease Priority Review Voucher (PRV) - 09 February ($110m)
BioMarin Pharmaceutical has entered into a definitive agreement with an undisclosed purchaser to sell the Rare Pediatric Disease Priority Review Voucher (PRV) it obtained in November 2021 for a lump sum payment of $110 million.
The company received the voucher under a Food and Drug Administration (FDA) program intended to encourage the development of treatments for rare pediatric diseases. BioMarin was awarded the voucher when it received approval of VOXZOGO (vosoritide) for Injection, indicated to increase linear growth in pediatric patients with achondroplasia five years of age and older with open epiphyses (growth plates).
The transaction remains subject to customary closing conditions, including anti-trust review.
Dr. Reddy's Laboratories to Acquire Cidmus from Novartis - 02 April ($61m)
Dr. Reddy's Laboratories Limited has entered into an agreement with Novartis AG, a pharmaceutical corporation, to acquire the cardiovascular brand Cidmus in India.
Under the agreement, Dr. Reddy's will be assigned and transferred the cidmus trademark in India from Novartis AG, for a consideration of $61 million (INR465.3 crore).
The Cidmus brand shall be affixed on the pharmaceutical composition comprising a combination of Valsartan and Sacubitril (currently under Novartis patent) which is indicated for heart failure patients with reduced ejection fraction. The tablets are available in three strengths.
As per IQVIA MAT, Cidmus saw sales of Rs136.4 crore in India for the most recent twelve months ending in February 2022. Dr. Reddy's will look to leverage its wide base to engage with healthcare professionals, and to significantly enhance the reach of the product in and beyond metros into tier-I and tier-II markets in India through its strong marketing and distribution network to maximise access to patients in need.
Further, the acquisition of Cidmus is yet another move by Dr. Reddy's in India to widen access of healthcare professionals and patients to well-established brands. Given the prevalence of cardiovascular diseases, this acquisition will allow Dr. Reddy's to make a trusted portfolio of medicines available to patients in India in keeping with its purpose of Good Health Can't Wait.
Cidmus will be a strong addition to the Dr. Reddy's existing portfolio in the cardiovascular segment alongside its leading brands such as Stamlo, Stamlo Beta, Reclide-XR and Reclimet-XR, and will take it closer to its ambition of breaking into the top 10 cardiac players in the Indian pharmaceutical market. It will also strengthen the presence of Dr. Reddy's in the chronic space in India as its India business continues to be a solid growth driver and focus market.
The agreement follows an exclusive sales and distribution agreement announced in february as part of which Dr. Reddy's will promote and distribute select Novartis products, including the voveran range, the Calcium range and Methergine in India.
Lotus Pharma to Acquire Cialis (Tadalafil) from Eli Lilly - 22 March ($58m)
Lotus Pharmaceutical Co, a generic pharmaceutical company, has agreed to acquire trademark, marketing authorization, and manufacturing know-how of Tadalafil 2.5mg, 5mg, 10mg and 20mg under the brand name of Cialis in Taiwan, from Eli Lilly and Co for USD57.5 million.
Tadalafil is indicated for the treatment of erectile dysfunction and benign prostatic hyperplasia, and Cialis is positioned as the top 2 brand product for erectile dysfunction treatment with high brand loyalty.
Immediately upon closing the transaction, the company will take over the Cialis business.
The completion of this transaction is subject to customary closing conditions.
Jazz Pharma to Sell Sunosi (solriamfetol) to Axsome Therapeutics - 28 March ($53m)
Jazz Pharmaceuticals, has entered into a definitive agreement to sell Sunosi (solriamfetol), a dual-acting dopamine and norepinephrine reuptake inhibitor shown to improve wakefulness in adults living with excessive daytime sleepiness (EDS) due to narcolepsy or obstructive sleep apnea (OSA), to Axsome Therapeutics, Inc, a biopharmaceutical company.
Under the terms of the agreement, Axsome will receive the rights to Sunosi in all of the existing territories available to Jazz. Jazz will receive attractive financial terms including an upfront payment of USD53 million, a high single-digit royalty on Axsome's U.S. Net sales of Sunosi in current indications and a mid-single-digit royalty on Axsome's U.S. Net sales of Sunosi in future indications.
The transaction will enable Jazz to sharpen its focus on its highest strategic priorities designed to deliver sustainable growth and enhanced shareholder value.
Guggenheim Securities is acting as financial advisor and Cooley LLP is acting as legal counsel to Jazz. DLA Piper LLP is acting as legal counsel to Axsome.
The transaction is structured to be completed in sequential closings for the U.S. And ex-U.S. Territories. Subject to the satisfaction or waiver of the closing conditions, the companies expect the U.S. Transaction to close in the second quarter of 2022 and the ex-U.S. Transaction close to occur within 60 days following the close of the U.S. Transaction.

