Allergan has issued a voluntary, US-wide recall of its Taytulla oral contraceptives after placebo and active capsules were found to have been placed in the incorrect order in sample packs.
Reported by a physician, the packaging error covers sample packs from lot 5620706 with an expiration date of May 2019 distributed since 27 August 2017.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
As the medicine is indicated to prevent pregnancy, this misplacement of the first four active capsules with non-hormonal placebos creates a potential risk of unintended pregnancy.
A statement by Allergan read: “The reversing of the order may not be apparent to either new users or previous users of the product, increasing the likelihood of taking the capsules out of order. If patients have concerns regarding the possibility of an unintended pregnancy they should consult their physician.”
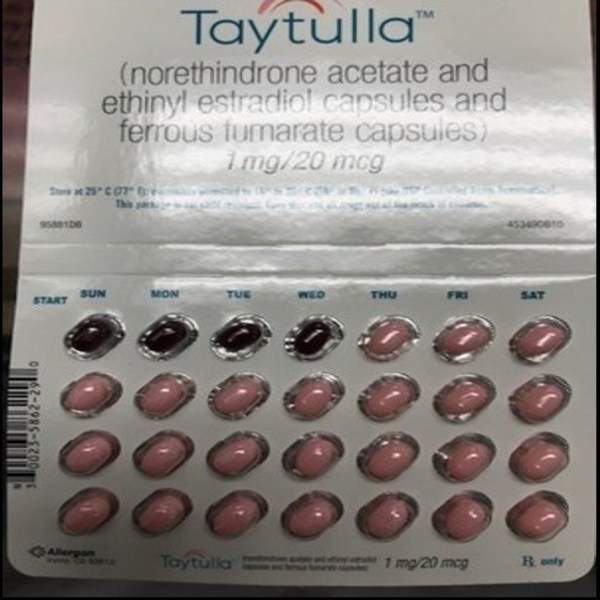
Taytulla is formulated using norethindrone acetate, ethinyl estradiol and ferrous fumarate capsules.
A Taytulla pill pack comes as a 28 count blister card and comprises 24 active hormone-containing pink softgel capsules to be taken for 24 days, along with four maroon softgel capsules (without hormones) to be used during the next four days.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataAllergan noted that the US Food and Drug Administration (FDA) is aware of the recall.
The company is making necessary arrangements for return of all sample packs associated with the lot number and has advised customers with these products to notify their physician in order to arrange a return.




