
Actelion Pharmaceuticals US, a Janssen Pharmaceutical Company of Johnson & Johnson, has received US Food and Drug Administration (FDA) approval for Tracleer (bosentan) to be used in paediatric patients with idiopathic or congenital pulmonary arterial hypertension (PAH).

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The FDA has granted approval for a new 32mg tablet for oral suspension for Tracleer to treat PAH patients aged three and older.
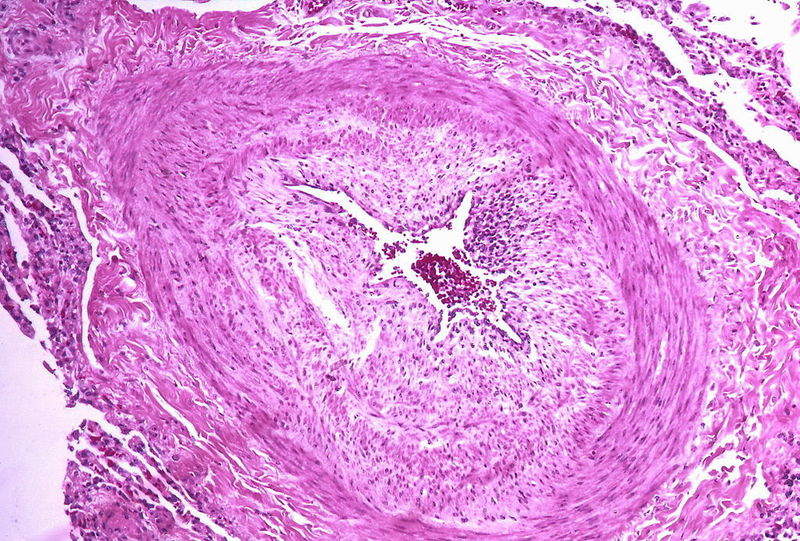
PAH is a chronic and progressive disease that results in abnormally high blood pressure in the arteries between the heart and lungs of an affected person.
The life-threatening disorder does not allow the blood to flow normally through the lungs and forces the patient’s heart to work harder.
Tracleer is an orally active endothelin receptor antagonist (ERA) that can be used to treat PAH patients by blocking the effects of the extra endothelin produced by their bodies.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe therapy has the potential to improve pulmonary vascular resistance (PVR), which is expected to improve the patient’s exercising ability.
Actelion Pharmaceuticals US Medical senior vice-president Dr Gary Palmer said: “Actelion has focused on the needs of the PAH community since Tracleer, our first treatment for PAH, was approved in 2001.
“We’re pleased our portfolio of treatments continues to grow and paediatric PAH patients will now have an FDA-approved treatment option available.”
The Actelion medicine is currently the first treatment approved by the FDA for paediatric PAH patients in the US.
The company expects to make the 32mg oral treatment available by the fourth quarter of this year.
Image: Micrograph showing arteries in pulmonary hypertensive with marked thickening of the walls. Photo: courtesy of Yale Rosen from USA.




